Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system
- PMID: 20399573
- PMCID: PMC2908725
- DOI: 10.1016/j.ijrobp.2009.12.061
Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system
Erratum in
- Int J Radiat Oncol Biol Phys. 2012 Sep 1;84(1):6. Grewal, Jai [added]
Abstract
Purpose: To conduct a controlled trial of bevacizumab for the treatment of symptomatic radiation necrosis of the brain.
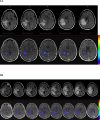
Methods and materials: A total of 14 patients were entered into a placebo-controlled randomized double-blind study of bevacizumab for the treatment of central nervous system radiation necrosis. All patients were required to have radiographic or biopsy proof of central nervous system radiation necrosis and progressive neurologic symptoms or signs. Eligible patients had undergone irradiation for head-and-neck carcinoma, meningioma, or low- to mid-grade glioma. Patients were randomized to receive intravenous saline or bevacizumab at 3-week intervals. The magnetic resonance imaging findings 3 weeks after the second treatment and clinical signs and symptoms defined the response or progression.
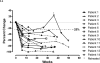
Results: The volumes of necrosis estimated on T(2)-weighted fluid-attenuated inversion recovery and T(1)-weighted gadolinium-enhanced magnetic resonance imaging scans demonstrated that although no patient receiving placebo responded (0 of 7), all bevacizumab-treated patients did so (5 of 5 randomized and 7 of 7 crossover) with decreases in T(2)-weighted fluid-attenuated inversion recovery and T(1)-weighted gadolinium-enhanced volumes and a decrease in endothelial transfer constant. All bevacizumab-treated patients-and none of the placebo-treated patients-showed improvement in neurologic symptoms or signs. At a median of 10 months after the last dose of bevacizumab in patients receiving all four study doses, only 2 patients had experienced a recurrence of magnetic resonance imaging changes consistent with progressive radiation necrosis; one patient received a single additional dose of bevacizumab and the other patient received two doses.
Conclusion: The Class I evidence of bevacizumab efficacy from the present study in the treatment of central nervous system radiation necrosis justifies consideration of this treatment option for people with radiation necrosis secondary to the treatment of head-and-neck cancer and brain cancer.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Cheung M-C, Chan AS, Law SC, et al. Impact of radionecrosis on cognitive dysfunction in patients after radiotherapy for nasopharyngeal carcinoma. Cancer. 2003;97:2019–2026. - PubMed
-
- Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurolog. 2003;9:180–188. - PubMed
-
- Crossen JR, Garwood D, Glatstein E, et al. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. Journal of Clinical Oncology. 1994;12:627–642. - PubMed
-
- Leibel SA, Sheline GE. Tolerance of the brain and spinal cord to conventional irradiation. In: PH G, SA L, GE S, editors. Radiation injury to the nervous system. Raven Press; New York: 1991. pp. 211–239.
-
- Levin VA, Leibel SA, Gutin PH. Neoplasms of the central nervous system. In: DeVita VTJ, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6 ed. Lippincott-Raven; Philadelphia: 2001. pp. 2100–2160.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

