Centrosomal localization of cyclin E-Cdk2 is required for initiation of DNA synthesis
- PMID: 20399658
- PMCID: PMC2897751
- DOI: 10.1016/j.cub.2010.03.028
Centrosomal localization of cyclin E-Cdk2 is required for initiation of DNA synthesis
Abstract
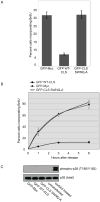
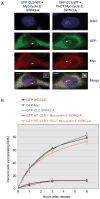
Cyclin E-Cdk2 is known to regulate both DNA replication and centrosome duplication during the G1-S transition in the cell cycle, and disruption of centrosomes results in a G1 arrest in some cell types. Localization of cyclin E on centrosomes is mediated by a 20 amino acid domain termed the centrosomal localization sequence (CLS), and expression of the GFP-tagged CLS displaces both cyclin E and cyclin A from the centrosome. In asynchronous cells, CLS expression inhibits the incorporation of bromodeoxyuridine (BrdU) into DNA, an effect proposed to reflect a G1 arrest. Here we show in synchronized cells that the reduction in BrdU incorporation reflects not a G1 arrest but rather direct inhibition of the initiation of DNA replication in S phase. The loading of essential DNA replication factors such as Cdc45 and proliferating cell nuclear antigen onto chromatin is blocked by CLS expression, but DNA synthesis can be rescued by retargeting active cyclin E-Cdk2 to the centrosome. These results suggest that initial steps of DNA replication require centrosomally localized Cdk activity and link the nuclear cycle with the centrosome cycle at the G1-S transition.
(c) 2010 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Centrosomal localization of cyclins E and A: structural similarities and functional differences.Cell Cycle. 2011 Jan 15;10(2):199-205. doi: 10.4161/cc.10.2.14444. Epub 2011 Jan 15. Cell Cycle. 2011. PMID: 21217199 Free PMC article.
-
Cyclin E-dependent localization of MCM5 regulates centrosome duplication.J Cell Sci. 2008 Oct 1;121(Pt 19):3224-32. doi: 10.1242/jcs.034702. J Cell Sci. 2008. PMID: 18799789
-
A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry.Science. 2004 Oct 29;306(5697):885-8. doi: 10.1126/science.1103544. Science. 2004. PMID: 15514162
-
Activation of Cdk2/Cyclin E complexes is dependent on the origin of replication licensing factor Cdc6 in mammalian cells.Cell Cycle. 2010 Nov 15;9(22):4533-41. doi: 10.4161/cc.9.22.13789. Epub 2010 Nov 15. Cell Cycle. 2010. PMID: 21088490
-
The role of nucleophosmin in centrosome duplication.Oncogene. 2002 Sep 9;21(40):6170-4. doi: 10.1038/sj.onc.1205708. Oncogene. 2002. PMID: 12214246 Review.
Cited by
-
Phosphorylation of the prolyl isomerase Cyclophilin A regulates its localisation and release from the centrosome during mitosis.Cell Cycle. 2023 Apr;22(8):951-966. doi: 10.1080/15384101.2023.2167430. Epub 2023 Jan 23. Cell Cycle. 2023. PMID: 36691345 Free PMC article.
-
Cell cycle regulation and anticancer drug discovery.Cancer Biol Med. 2017 Nov;14(4):348-362. doi: 10.20892/j.issn.2095-3941.2017.0033. Cancer Biol Med. 2017. PMID: 29372101 Free PMC article.
-
Cell cycle, cytoskeleton dynamics and beyond: the many functions of cyclins and CDK inhibitors.Cell Cycle. 2015;14(12):1786-98. doi: 10.1080/15384101.2014.998085. Cell Cycle. 2015. PMID: 25789852 Free PMC article. Review.
-
γ-Tubulin plays a key role in inactivating APC/C(Cdh1) at the G(1)-S boundary.J Cell Biol. 2012 Sep 3;198(5):785-91. doi: 10.1083/jcb.201203115. Epub 2012 Aug 27. J Cell Biol. 2012. PMID: 22927465 Free PMC article.
-
γ-Tubulin⁻γ-Tubulin Interactions as the Basis for the Formation of a Meshwork.Int J Mol Sci. 2018 Oct 19;19(10):3245. doi: 10.3390/ijms19103245. Int J Mol Sci. 2018. PMID: 30347727 Free PMC article. Review.
References
-
- Hinchcliffe EH, Li C, Thompson EA, Maller JL, Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science. 1999;283:851–854. - PubMed
-
- Matsumoto Y, Hayashi K, Nishida E. Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr Biol. 1999;9:429–432. - PubMed
-
- Coverley D, Laman H, Laskey RA. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat Cell Biol. 2002;4:523–528. - PubMed
-
- Hinchcliffe EH, Miller FJ, Cham M, Khodjakov A, Sluder G. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science. 2001;291:1547–1550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

