The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein
- PMID: 20399660
- PMCID: PMC2921989
- DOI: 10.1016/j.tcb.2010.03.004
The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein
Abstract
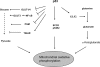
In response to stress, p53 initiates the transcriptional regulation of selected target genes and various cellular responses, including cell cycle arrest, apoptosis and senescence. Recent studies revealed two additional functions of p53 in the regulation of IGF-1/AKT/mTOR pathways and energy metabolism, contributing to p53's role as a tumor suppressor. Oncogenic processes give rise to metabolic pathways focused upon the use of aerobic glycolysis (the Warburg effect) and the pentose shunt, providing higher levels of reducing activities. p53 shuts down these pathways and refocuses cells to utilize mitochondrial oxidative phosphorylation, thereby maximizing efficient ATP production and minimizing the synthesis of substrates for cell division. The use of these alternative metabolic pathways is an integral part of both normal and oncogenic phenotypes.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

References
-
- Levine AJ, et al. The P53 pathway: what questions remain to be explored? Cell Death Differ. 2006;13:1027–1036. - PubMed
-
- Levine AJ, et al. P53 is a tumor suppressor gene. Cell. 2004;116:S67–69. 61 p following S69. - PubMed
-
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. - PubMed
-
- Tanaka H, et al. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature. 2000;404:42–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

