Design principles of insect and vertebrate visual systems
- PMID: 20399726
- PMCID: PMC2871012
- DOI: 10.1016/j.neuron.2010.01.018
Design principles of insect and vertebrate visual systems
Abstract
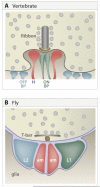
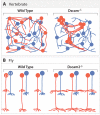
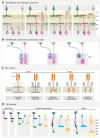
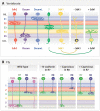
A century ago, Cajal noted striking similarities between the neural circuits that underlie vision in vertebrates and flies. Over the past few decades, structural and functional studies have provided strong support for Cajal's view. In parallel, genetic studies have revealed some common molecular mechanisms controlling development of vertebrate and fly visual systems and suggested that they share a common evolutionary origin. Here, we review these shared features, focusing on the first several layers-retina, optic tectum (superior colliculus), and lateral geniculate nucleus in vertebrates; and retina, lamina, and medulla in fly. We argue that vertebrate and fly visual circuits utilize common design principles and that taking advantage of this phylogenetic conservation will speed progress in elucidating both functional strategies and developmental mechanisms, as has already occurred in other areas of neurobiology ranging from electrical signaling and synaptic plasticity to neurogenesis and axon guidance.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures







References
-
- Agathocleous M, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Annu Rev Cell Dev Biol. 2009;25:45–69. - PubMed
-
- Bazigou E, Apitz H, Johansson J, Loren CE, Hirst EM, Chen PL, Palmer RH, Salecker I. Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell. 2007;128:961–975. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

