Cranial neural crest migration: new rules for an old road
- PMID: 20399765
- PMCID: PMC2914193
- DOI: 10.1016/j.ydbio.2010.04.010
Cranial neural crest migration: new rules for an old road
Abstract
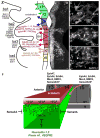
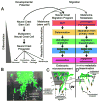
The neural crest serve as an excellent model to better understand mechanisms of embryonic cell migration. Cell tracing studies have shown that cranial neural crest cells (CNCCs) emerge from the dorsal neural tube in a rostrocaudal manner and are spatially distributed along stereotypical, long distance migratory routes to precise targets in the head and branchial arches. Although the CNCC migratory pattern is a beautifully choreographed and programmed invasion, the underlying orchestration of molecular events is not well known. For example, it is still unclear how single CNCCs react to signals that direct their choice of direction and how groups of CNCCs coordinate their interactions to arrive at a target in an ordered manner. In this review, we discuss recent cellular and molecular discoveries of the CNCC migratory pattern. We focus on events from the time when CNCCs encounter the tissue adjacent to the neural tube and their travel through different microenvironments and into the branchial arches. We describe the patterning of discrete cell migratory streams that emerge from the hindbrain, rhombomere (r) segments r1-r7, and the signals that coordinate directed migration. We propose a model that attempts to unify many complex events that establish the CNCC migratory pattern, and based on this model we integrate information between cranial and trunk neural crest development.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

References
-
- Abbott DE, Postovit LM, Seftor EA, Margaryan NV, Seftor RE, Hendrix MJ. Exploiting the convergence of embryonic and tumorigenic signaling pathways to develop new therapeutic targets. Stem Cell Rev. 2007;3:68–78. - PubMed
-
- Abercrombie M, Heaysman JE. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp Cell Res. 1953;5:111–31. - PubMed
-
- Abercrombie M, Heaysman JE. Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp Cell Res. 1954;6:293–306. - PubMed
-
- Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

