The role of CO(2) and central chemoreception in the control of breathing in the fetus and the neonate
- PMID: 20399912
- PMCID: PMC2988425
- DOI: 10.1016/j.resp.2010.04.009
The role of CO(2) and central chemoreception in the control of breathing in the fetus and the neonate
Abstract
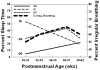
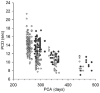
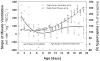
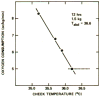
Central chemoreception is active early in development and likely drives fetal breathing movements, which are influenced by a combination of behavioral state and powerful inhibition. In the premature human infant and newborn rat ventilation increases in response to CO(2); in the rat the sensitivity of the response increases steadily after ∼P12. The premature human infant is more vulnerable to instability than the newborn rat and exhibits periodic breathing that is augmented by hypoxia and eliminated by breathing oxygen or CO(2) or the administration of respiratory stimulants. The sites of central chemoreception active in the fetus are not known, but may involve the parafacial respiratory group which may be a precursor to the adult RTN. The fetal and neonatal rat brainstem-spinal-cord preparations promise to provide important information about central chemoreception in the developing rodent and will increase our understanding of important clinical problems, including The Sudden Infant Death Syndrome, Congenital Central Hypoventilation Syndrome, and periodic breathing and apnea of prematurity.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures



References
-
- Abu-Shaweesh JM, Dreshaj IA, Thomas AJ, Haxhiu MA, Strohl KP, Martin RJ. Changes in respiratory timing induced by hypercapnia in maturing rats. J Appl Physiol. 1999;87:484–490. - PubMed
-
- Adamson SL, Kuipers IM, Olson DM. Umbilical cord occlusion stimulates breathing independent of blood gases and pH. J Appl Physiol. 1991;70:1796–1809. - PubMed
-
- Aizenfisz S, Dauger S, Durand E, Vardon G, Levacher B, Simonneau M, Pachnis V, Gaultier C, Gallego J. Ventilatory responses to hypercapnia and hypoxia in heterozygous c-ret newborn mice. Resp Physiol & Neurobiol. 2002;131:213–222. - PubMed
-
- Albersheim S, Boychuk R, Seshia MM, Cates D, Rigatto H. Effects of CO2 on immediate ventilatory response to O2 in preterm infants. J Appl Physiol. 1976;41:609–611. - PubMed
-
- Alvarez JE, Bodani J, Fajardo CA, Kwiatkowski K, Cates DB, Rigatto H. Sighs and their relationship to apnea in the newborn infant. Biol Neon. 1993;63:139–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

