OX40 induces CCL20 expression in the context of antigen stimulation: an expanding role of co-stimulatory molecules in chemotaxis
- PMID: 20400327
- PMCID: PMC2867600
- DOI: 10.1016/j.cyto.2010.03.021
OX40 induces CCL20 expression in the context of antigen stimulation: an expanding role of co-stimulatory molecules in chemotaxis
Abstract
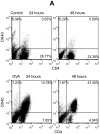
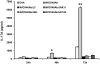
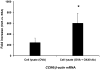
OX40 is an inducible co-stimulatory molecule expressed by activated T cells. It plays an important role in the activation and proliferation of T lymphocytes. Recently, some co-stimulatory molecules have been shown to direct leukocyte trafficking. Chemotaxis is essential for achieving an effective immune response. CCL20 is an important chemoattractant produced by activated T cells. In this study, using DO11.10 mice whose transgenic T cell receptor specifically recognizes ovalbumin, we demonstrate that ovalbumin induces OX40 expression in CD4+ lymphocytes. Further stimulation of OX40 by OX40 activating antibody up-regulates CCL20 production. Both NF-kappaB dependent and independent signaling pathways are implicated in the induction of CCL20 by OX40. Finally, we primed the DO11.10 splenocytes with or without OX40 activating antibody in the presence of ovalbumin. Intranasal administration of the cell lysates derived from the cells with OX40 stimulation results in more severe leukocyte infiltration in the lung of DO11.10 mice, which is substantially attenuated by CCL20 blocking antibody. Taken together, this study has shown that activation of OX40 induces CCL20 expression in the presence of antigen stimulation. Thus, our results broaden the role of OX40 in chemotaxis, and reveal a novel effect of co-stimulatory molecules in orchestrating both T cell up-regulation and migration.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Roscovitine suppresses CD4+ T cells and T cell-mediated experimental uveitis.PLoS One. 2013 Nov 18;8(11):e81154. doi: 10.1371/journal.pone.0081154. eCollection 2013. PLoS One. 2013. PMID: 24260551 Free PMC article.
-
Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival.J Immunol. 2008 Jun 1;180(11):7240-8. doi: 10.4049/jimmunol.180.11.7240. J Immunol. 2008. PMID: 18490723 Free PMC article.
-
Requirements for the functional expression of OX40 ligand on human activated CD4+ and CD8+ T cells.Hum Immunol. 2007 Jul;68(7):563-71. doi: 10.1016/j.humimm.2007.03.012. Epub 2007 Apr 13. Hum Immunol. 2007. PMID: 17584577
-
Science gone translational: the OX40 agonist story.Immunol Rev. 2011 Nov;244(1):218-31. doi: 10.1111/j.1600-065X.2011.01069.x. Immunol Rev. 2011. PMID: 22017441 Free PMC article. Review.
-
The role of OX40-mediated co-stimulation in T-cell activation and survival.Crit Rev Immunol. 2009;29(3):187-201. doi: 10.1615/critrevimmunol.v29.i3.10. Crit Rev Immunol. 2009. PMID: 19538134 Free PMC article. Review.
Cited by
-
FcγRIIB engagement drives agonistic activity of Fc-engineered αOX40 antibody to stimulate human tumor-infiltrating T cells.J Immunother Cancer. 2020 Sep;8(2):e000816. doi: 10.1136/jitc-2020-000816. J Immunother Cancer. 2020. PMID: 32900860 Free PMC article.
-
Intraperitoneal administration of the anti-IL-23 antibody prevents the establishment of intestinal nematodes in mice.Sci Rep. 2018 May 17;8(1):7787. doi: 10.1038/s41598-018-26194-x. Sci Rep. 2018. PMID: 29773890 Free PMC article.
-
15 kDa Granulysin versus GM-CSF for monocytes differentiation: analogies and differences at the transcriptome level.J Transl Med. 2011 Apr 18;9:41. doi: 10.1186/1479-5876-9-41. J Transl Med. 2011. PMID: 21501511 Free PMC article.
-
Defining the Signature of VISTA on Myeloid Cell Chemokine Responsiveness.Front Immunol. 2019 Nov 19;10:2641. doi: 10.3389/fimmu.2019.02641. eCollection 2019. Front Immunol. 2019. PMID: 31803182 Free PMC article.
References
-
- Mosmann TR, Li L, Hengartner H, Kagi D, Fu W, Sad S. Differentiation and functions of T cell subsets. Ciba Found Sym. 1997;204:148–54. - PubMed
-
- Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat Immunol. 2001;2(6):487–92. - PubMed
-
- Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009;123(5):1004–11. - PubMed
-
- Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

