TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration
- PMID: 20400460
- PMCID: PMC3167692
- DOI: 10.1093/hmg/ddq137
TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration
Abstract
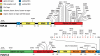
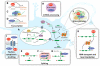
Amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) are neurodegenerative diseases with clinical and pathological overlap. Landmark discoveries of mutations in the transactive response DNA-binding protein (TDP-43) and fused in sarcoma/translocated in liposarcoma (FUS/TLS) as causative of ALS and FTLD, combined with the abnormal aggregation of these proteins, have initiated a shifting paradigm for the underlying pathogenesis of multiple neurodegenerative diseases. TDP-43 and FUS/TLS are both RNA/DNA-binding proteins with striking structural and functional similarities. Their association with ALS and other neurodegenerative diseases is redirecting research efforts toward understanding the role of RNA processing regulation in neurodegeneration.
Figures
References
-
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J.P., Deng H.X., et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi:10.1038/362059a0. - DOI - PubMed
-
- Boillee S., Vande Velde C., Cleveland D.W. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi:10.1016/j.neuron.2006.09.018. - DOI - PubMed
-
- Pasinelli P., Brown R.H. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat. Rev. Neurosci. 2006;7:710–723. doi:10.1038/nrn1971. - DOI - PubMed
-
- Valdmanis P.N., Rouleau G.A. Genetics of familial amyotrophic lateral sclerosis. Neurology. 2008;70:144–152. doi:10.1212/01.wnl.0000296811.19811.db. - DOI - PubMed
-
- Ilieva H., Polymenidou M., Cleveland D.W. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell. Biol. 2009;187:761–772. doi:10.1083/jcb.200908164. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

