Birefringence and DNA condensation of liquid crystalline chromosomes
- PMID: 20400466
- PMCID: PMC2950428
- DOI: 10.1128/EC.00026-10
Birefringence and DNA condensation of liquid crystalline chromosomes
Abstract
DNA can self-assemble in vitro into several liquid crystalline phases at high concentrations. The largest known genomes are encoded by the cholesteric liquid crystalline chromosomes (LCCs) of the dinoflagellates, a diverse group of protists related to the malarial parasites. Very little is known about how the liquid crystalline packaging strategy is employed to organize these genomes, the largest among living eukaryotes-up to 80 times the size of the human genome. Comparative measurements using a semiautomatic polarizing microscope demonstrated that there is a large variation in the birefringence, an optical property of anisotropic materials, of the chromosomes from different dinoflagellate species, despite their apparently similar ultrastructural patterns of bands and arches. There is a large variation in the chromosomal arrangements in the nuclei and individual karyotypes. Our data suggest that both macroscopic and ultrastructural arrangements affect the apparent birefringence of the liquid crystalline chromosomes. Positive correlations are demonstrated for the first time between the level of absolute retardance and both the DNA content and the observed helical pitch measured from transmission electron microscopy (TEM) photomicrographs. Experiments that induced disassembly of the chromosomes revealed multiple orders of organization in the dinoflagellate chromosomes. With the low protein-to-DNA ratio, we propose that a highly regulated use of entropy-driven force must be involved in the assembly of these LCCs. Knowledge of the mechanism of packaging and arranging these largest known DNAs into different shapes and different formats in the nuclei would be of great value in the use of DNA as nanostructural material.
Figures
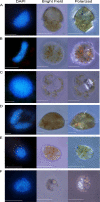

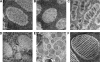

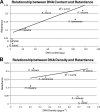

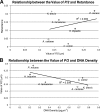
References
-
- Bennett M., Wong J. T. Y. 2006. Evolution and diversity of dinoflagellates: molecular perspectives, p89–115InSharma A. K., Sharma A.(ed.), Plant genome: biodiversity and evolution, vol. 2B Lower groups. Science Publishers, Enfield, NH
-
- Bohrmann B., Haider M., Kellenberger E. 1993. Concentration evaluation of chromatin in unstained resin-embedded sections by means of low-dose ratio-contrast imaging in STEM. Ultramicroscopy 49:235–251 - PubMed
-
- Bouligand Y., Norris V. 2001. Chromosome separation and segregation in dinoflagellates and bacteria may depend on liquid crystalline states. Biochimie 83:187–192 - PubMed
-
- Bouligand Y., Soyer M.-O., Puiseux-Dao S. 1968. The fibrillary structure and orientation of chromosomes in dinoflagellata. Chromosoma 24:251–287 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

