Isocitrate dehydrogenase is important for nitrosative stress resistance in Cryptococcus neoformans, but oxidative stress resistance is not dependent on glucose-6-phosphate dehydrogenase
- PMID: 20400467
- PMCID: PMC2901651
- DOI: 10.1128/EC.00271-09
Isocitrate dehydrogenase is important for nitrosative stress resistance in Cryptococcus neoformans, but oxidative stress resistance is not dependent on glucose-6-phosphate dehydrogenase
Abstract
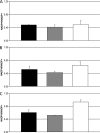
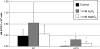
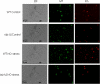
The opportunistic intracellular fungal pathogen Cryptococcus neoformans depends on many antioxidant and denitrosylating proteins and pathways for virulence in the immunocompromised host. These include the glutathione and thioredoxin pathways, thiol peroxidase, cytochrome c peroxidase, and flavohemoglobin denitrosylase. All of these ultimately depend on NADPH for either catalytic activity or maintenance of a reduced, functional form. The need for NADPH during oxidative stress is well established in many systems, but a role in resistance to nitrosative stress has not been as well characterized. In this study we investigated the roles of two sources of NADPH, glucose-6-phosphate dehydrogenase (Zwf1) and NADP(+)-dependent isocitrate dehydrogenase (Idp1), in production of NADPH and resistance to oxidative and nitrosative stress. Deletion of ZWF1 in C. neoformans did not result in an oxidative stress sensitivity phenotype or changes in the amount of NADPH produced during oxidative stress compared to those for the wild type. Deletion of IDP1 resulted in greater sensitivity to nitrosative stress than to oxidative stress. The amount of NADPH increased 2-fold over that in the wild type during nitrosative stress, and yet the idp1Delta strain accumulated more mitochondrial damage than the wild type during nitrosative stress. This is the first report of the importance of Idp1 and NADPH for nitrosative stress resistance.
Figures




Similar articles
-
Cytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cells.Free Radic Biol Med. 2002 Jun 1;32(11):1185-96. doi: 10.1016/s0891-5849(02)00815-8. Free Radic Biol Med. 2002. PMID: 12031902
-
Antioxidant function of cytosolic sources of NADPH in yeast.Free Radic Biol Med. 2001 Sep 15;31(6):832-43. doi: 10.1016/s0891-5849(01)00666-9. Free Radic Biol Med. 2001. PMID: 11557322
-
Activation of NADPH-recycling systems in leaves and roots of Arabidopsis thaliana under arsenic-induced stress conditions is accelerated by knock-out of Nudix hydrolase 19 (AtNUDX19) gene.J Plant Physiol. 2016 Mar 15;192:81-9. doi: 10.1016/j.jplph.2016.01.010. Epub 2016 Feb 3. J Plant Physiol. 2016. PMID: 26878367
-
Purification and characterization of glucose-6-phosphate dehydrogenase from Cryptococcus neoformans: identification as "nothing dehydrogenase".Arch Biochem Biophys. 1994 Sep;313(2):304-9. doi: 10.1006/abbi.1994.1392. Arch Biochem Biophys. 1994. PMID: 8080277
-
The roles of NADPH and isocitrate dehydrogenase in cochlear mitochondrial antioxidant defense and aging.Hear Res. 2023 Jan;427:108659. doi: 10.1016/j.heares.2022.108659. Epub 2022 Nov 24. Hear Res. 2023. PMID: 36493529 Free PMC article. Review.
Cited by
-
Proteomic profiling of Rhizobium tropici PRF 81: identification of conserved and specific responses to heat stress.BMC Microbiol. 2012 May 30;12:84. doi: 10.1186/1471-2180-12-84. BMC Microbiol. 2012. PMID: 22647150 Free PMC article.
-
Global transcriptome profile of Cryptococcus neoformans during exposure to hydrogen peroxide induced oxidative stress.PLoS One. 2013;8(1):e55110. doi: 10.1371/journal.pone.0055110. Epub 2013 Jan 28. PLoS One. 2013. PMID: 23383070 Free PMC article.
-
One-step metabolomics: carbohydrates, organic and amino acids quantified in a single procedure.J Vis Exp. 2010 Jun 25;(40):2014. doi: 10.3791/2014. J Vis Exp. 2010. PMID: 20613709 Free PMC article.
-
Effect of cadmium toxicity on growth, physiochemical parameters and antioxidant system of castor seedlings.Heliyon. 2024 Aug 20;10(16):e36536. doi: 10.1016/j.heliyon.2024.e36536. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39262939 Free PMC article.
-
What Are the Best Parents for Hybrid Progeny? An Investigation into the Human Pathogenic Fungus Cryptococcus.J Fungi (Basel). 2021 Apr 15;7(4):299. doi: 10.3390/jof7040299. J Fungi (Basel). 2021. PMID: 33920829 Free PMC article.
References
-
- Alvarez M., Casadevall A. 2006. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 16:2161–2165 - PubMed
-
- Ayene I. S., Stamato T. D., Mauldin S. K., Biaglow J. E., Tuttle S. W., Jenkins S. F., Koch C. J. 2002. Mutation in the glucose-6-phosphate dehydrogenase gene leads to inactivation of Ku DNA end binding during oxidative stress. J. Biol. Chem. 277:9929–9935 - PubMed
-
- Boveris A., Cadenas E. 1982. Production of superoxide radicals and hydrogen peroxide in mitochondria, p. 15–30InOberley L. W. (ed.), Superoxide dismutase, vol. III CRC Press, Boca Raton, FL
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

