Cbl-b is a novel physiologic regulator of glycoprotein VI-dependent platelet activation
- PMID: 20400514
- PMCID: PMC2878491
- DOI: 10.1074/jbc.M109.080200
Cbl-b is a novel physiologic regulator of glycoprotein VI-dependent platelet activation
Abstract
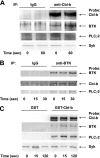
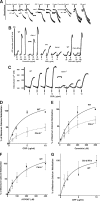
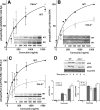
Cbl-b, a member of the Cbl family of E3 ubiquitin ligases, plays an important role in the activation of lymphocytes. However, its function in platelets remains unknown. We show that Cbl-b is expressed in human platelets along with c-Cbl, but in contrast to c-Cbl, it is not tyrosine-phosphorylated upon glycoprotein VI (GPVI) stimulation. Cbl-b, unlike c-Cbl, is not required for Syk ubiquitylation downstream of GPVI activation. Phospholipase Cgamma2 (PLCgamma2) and Bruton's tyrosine kinase (BTK) are constituently associated with Cbl-b. Cbl-b-deficient (Cbl-b(-/-)) platelets display an inhibition in the concentration-response curve for GPVI-specific agonist-induced aggregation, secretion, and Ca(2+) mobilization. A parallel inhibition is found for activation of PLCgamma2 and BTK. However, Syk activation is not affected by the absence of Cbl-b, indicating that Cbl-b acts downstream of Syk but upstream of BTK and PLCgamma2. When Cbl-b(-/-) mice were tested in the ferric chloride thrombosis model, occlusion time was increased and clot stability was reduced compared with wild type controls. These data indicate that Cbl-b plays a positive modulatory role in GPVI-dependent platelet signaling, which translates to an important regulatory role in hemostasis and thrombosis in vivo.
Figures
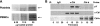


Similar articles
-
Bruton's Tyrosine Kinase mediates platelet receptor-induced generation of microparticles: a potential mechanism for amplification of inflammatory responses in rheumatoid arthritis synovial joints.Immunol Lett. 2013 Feb;150(1-2):97-104. doi: 10.1016/j.imlet.2012.12.007. Epub 2012 Dec 21. Immunol Lett. 2013. PMID: 23266841
-
Roles of Src-like adaptor protein 2 (SLAP-2) in GPVI-mediated platelet activation SLAP-2 and GPVI signaling.Thromb Res. 2010 Oct;126(4):e276-85. doi: 10.1016/j.thromres.2010.07.010. Epub 2010 Sep 15. Thromb Res. 2010. PMID: 20828795
-
Differential Regulation of GPVI-Induced Btk and Syk Activation by PKC, PKA and PP2A in Human Platelets.Int J Mol Sci. 2023 Apr 24;24(9):7776. doi: 10.3390/ijms24097776. Int J Mol Sci. 2023. PMID: 37175486 Free PMC article.
-
The Cbl family proteins: ring leaders in regulation of cell signaling.J Cell Physiol. 2006 Oct;209(1):21-43. doi: 10.1002/jcp.20694. J Cell Physiol. 2006. PMID: 16741904 Review.
-
E3 ubiquitin ligase Cbl-b in innate and adaptive immunity.Cell Cycle. 2014;13(12):1875-84. doi: 10.4161/cc.29213. Epub 2014 May 14. Cell Cycle. 2014. PMID: 24875217 Free PMC article. Review.
Cited by
-
Phosphorylation of (Ser 291) in the linker insert of Syk negatively regulates ITAM signaling in platelets.Platelets. 2024 Dec;35(1):2369766. doi: 10.1080/09537104.2024.2369766. Epub 2024 Jun 21. Platelets. 2024. PMID: 38904212
-
Tyrosine phosphorylated c-Cbl regulates platelet functional responses mediated by outside-in signaling.Blood. 2011 Nov 17;118(20):5631-40. doi: 10.1182/blood-2011-01-328807. Epub 2011 Oct 3. Blood. 2011. PMID: 21967979 Free PMC article.
-
Identification of genes, pathways and transcription factor-miRNA-target gene networks and experimental verification in venous thromboembolism.Sci Rep. 2021 Aug 11;11(1):16352. doi: 10.1038/s41598-021-95909-4. Sci Rep. 2021. PMID: 34381164 Free PMC article.
-
Ascorbic acid improves thrombotic function of platelets during living donor liver transplantation by modulating the function of the E3 ubiquitin ligases c-Cbl and Cbl-b.J Int Med Res. 2019 May;47(5):1856-1867. doi: 10.1177/0300060518817408. Epub 2019 Jan 7. J Int Med Res. 2019. PMID: 30614340 Free PMC article.
-
ELMO1 deficiency enhances platelet function.Blood Adv. 2019 Feb 26;3(4):575-587. doi: 10.1182/bloodadvances.2018016444. Blood Adv. 2019. PMID: 30787021 Free PMC article.
References
-
- Watson S. P., Gibbins J. ( 1998) Immunol. Today 19, 260– 264 - PubMed
-
- Watson S. P., Auger J. M., McCarty O. J., Pearce A. C. ( 2005) J. Thromb. Haemost. 3, 1752– 1762 - PubMed
-
- Blake T. J., Shapiro M., Morse H. C., 3rd, Langdon W. Y. ( 1991) Oncogene 6, 653– 657 - PubMed
-
- Keane M. M., Rivero-Lezcano O. M., Mitchell J. A., Robbins K. C., Lipkowitz S. ( 1995) Oncogene 10, 2367– 2377 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

