Catalytic reactions of the homogentisate prenyl transferase involved in plastoquinone-9 biosynthesis
- PMID: 20400515
- PMCID: PMC2881743
- DOI: 10.1074/jbc.M110.117929
Catalytic reactions of the homogentisate prenyl transferase involved in plastoquinone-9 biosynthesis
Abstract
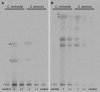
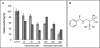
Homogentisate solanesyl transferase (HST) catalyzes the prenylation and decarboxylation of homogentisate to form 2-methyl-6-solanesyl-1,4-benzoquinol, the first intermediate in plastoquinone-9 biosynthesis. In vitro, HST from Spinacia oleracea L., Arabidopsis thaliana, and Chlamydomonas reinhardtii were all found to use not only solanesyl diphosphate but also short chain prenyl diphosphates of 10-20 carbon atoms as prenyl donors. Surprisingly, with these donors, prenyl transfer was largely decoupled from decarboxylation, and thus the major products were 6-prenyl-1,4-benzoquinol-2-methylcarboxylates rather than the expected 2-methyl-6-prenyl-1,4-benzoquinols. The 6-prenyl-1,4-benzoquinol-2-methylcarboxylates were not substrates for HST-catalyzed decarboxylation, and the enzyme kinetics associated with forming these products appeared quite distinct from those for 2-methyl-6-prenyl-1,4-benzoquinol formation in respect of catalytic rate, substrate K(m) value, and the pattern of inhibition by haloxydine, a molecule that appeared to act as a dead end mimic of homogentisate. These observations were reconciled into a simple model for the HST mechanism. Here, prenyl diphosphate binds to HST to form at least two alternative complexes that go on to react differently with homogentisate and prenylate it either with or without it first being decarboxylated. It is supposed that solanesyl diphosphate binds tightly and preferentially in the mode that compels prenylation with decarboxylation.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

