DNA binding by the ETS protein TEL (ETV6) is regulated by autoinhibition and self-association
- PMID: 20400516
- PMCID: PMC2881775
- DOI: 10.1074/jbc.M109.096958
DNA binding by the ETS protein TEL (ETV6) is regulated by autoinhibition and self-association
Abstract
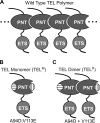
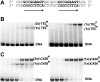
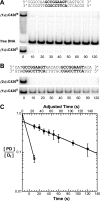
The ETS protein TEL, a transcriptional repressor, contains a PNT domain that, as an isolated fragment in vitro, self-associates to form a head-to-tail polymer. How such polymerization might affect the DNA-binding properties of full-length TEL is unclear. Here we report that monomeric TEL binds to a consensus ETS site with unusually low affinity (K(d) = 2.8 x 10(-8) M). A deletion analysis demonstrated that the low affinity was caused by a C-terminal inhibitory domain (CID) that attenuates DNA binding by approximately 10-fold. An NMR spectroscopically derived structure of a TEL fragment, deposited in the Protein Data Bank, revealed that the CID consists of two alpha-helices, one of which appears to block the DNA binding surface of the TEL ETS domain. Based on this structure, we substituted two conserved glutamic acids (Glu-431 and Glu-434) with alanines and found that this activated DNA binding and enhanced trypsin sensitivity in the CID. We propose that TEL displays a conformational equilibrium between inhibited and activated states and that electrostatic interactions involving these negatively charged residues play a role in stabilizing the inhibited conformation. Using a TEL dimer as a model polymer, we show that self-association facilitates cooperative binding to DNA. Cooperativity was observed on DNA duplexes containing tandem consensus ETS sites at variable spacing and orientations, suggesting flexibility in the region of TEL linking its self-associating PNT domain and DNA-binding ETS domain. We speculate that TEL compensates for the low affinity, which is caused by autoinhibition, by binding to DNA as a cooperative polymer.
Figures


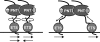
Similar articles
-
Autoinhibition of ETV6 DNA Binding Is Established by the Stability of Its Inhibitory Helix.J Mol Biol. 2016 Apr 24;428(8):1515-30. doi: 10.1016/j.jmb.2016.02.020. Epub 2016 Feb 23. J Mol Biol. 2016. PMID: 26920109 Free PMC article.
-
Autoinhibition of ETV6 (TEL) DNA binding: appended helices sterically block the ETS domain.J Mol Biol. 2012 Aug 3;421(1):67-84. doi: 10.1016/j.jmb.2012.05.010. Epub 2012 May 12. J Mol Biol. 2012. PMID: 22584210 Free PMC article.
-
Biophysical characterization of the ETV6 PNT domain polymerization interfaces.J Biol Chem. 2021 Jan-Jun;296:100284. doi: 10.1016/j.jbc.2021.100284. Epub 2021 Jan 13. J Biol Chem. 2021. PMID: 33450226 Free PMC article.
-
Proteins of the ETS family with transcriptional repressor activity.Oncogene. 2000 Dec 18;19(55):6524-32. doi: 10.1038/sj.onc.1204045. Oncogene. 2000. PMID: 11175368 Review.
-
Genomic and biochemical insights into the specificity of ETS transcription factors.Annu Rev Biochem. 2011;80:437-71. doi: 10.1146/annurev.biochem.79.081507.103945. Annu Rev Biochem. 2011. PMID: 21548782 Free PMC article. Review.
Cited by
-
Autoinhibition of ETV6 DNA Binding Is Established by the Stability of Its Inhibitory Helix.J Mol Biol. 2016 Apr 24;428(8):1515-30. doi: 10.1016/j.jmb.2016.02.020. Epub 2016 Feb 23. J Mol Biol. 2016. PMID: 26920109 Free PMC article.
-
Transcription factor mutations as a cause of familial myeloid neoplasms.J Clin Invest. 2019 Feb 1;129(2):476-488. doi: 10.1172/JCI120854. Epub 2019 Feb 1. J Clin Invest. 2019. PMID: 30707109 Free PMC article. Review.
-
Cooperative recruitment of Yan via a high-affinity ETS supersite organizes repression to confer specificity and robustness to cardiac cell fate specification.Genes Dev. 2018 Mar 1;32(5-6):389-401. doi: 10.1101/gad.307132.117. Epub 2018 Mar 13. Genes Dev. 2018. PMID: 29535190 Free PMC article.
-
Germline ETV6 mutations and predisposition to hematological malignancies.Int J Hematol. 2017 Aug;106(2):189-195. doi: 10.1007/s12185-017-2259-4. Epub 2017 May 29. Int J Hematol. 2017. PMID: 28555414 Review.
-
Region-specific regulation of 5-HT1A receptor expression by Pet-1-dependent mechanisms in vivo.J Neurochem. 2011 Mar;116(6):1066-76. doi: 10.1111/j.1471-4159.2010.07161.x. Epub 2011 Jan 24. J Neurochem. 2011. PMID: 21182526 Free PMC article.
References
-
- Tomlins S. A., Rhodes D. R., Perner S., Dhanasekaran S. M., Mehra R., Sun X. W., Varambally S., Cao X., Tchinda J., Kuefer R., Lee C., Montie J. E., Shah R. B., Pienta K. J., Rubin M. A., Chinnaiyan A. M. (2005) Science 310, 644–648 - PubMed
-
- Golub T. R., Barker G. F., Stegmaier K., Gilliland D. G. (1997) Curr. Top. Microbiol. Immunol. 220, 67–79 - PubMed
-
- Kwiatkowski B. A., Bastian L. S., Bauer T. R., Jr., Tsai S., Zielinska-Kwiatkowska A. G., Hickstein D. D. (1998) J. Biol. Chem. 273, 17525–17530 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

