The phosphoenolpyruvate phosphotransferase system regulates Vibrio cholerae biofilm formation through multiple independent pathways
- PMID: 20400550
- PMCID: PMC2901703
- DOI: 10.1128/JB.00213-10
The phosphoenolpyruvate phosphotransferase system regulates Vibrio cholerae biofilm formation through multiple independent pathways
Abstract
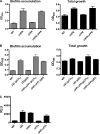
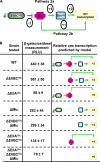
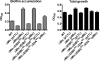
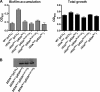
The bacterial phosphoenolpyruvate phosphotransferase system (PTS) is a highly conserved phosphotransfer cascade that participates in the transport and phosphorylation of selected carbohydrates and modulates many cellular functions in response to carbohydrate availability. It plays a role in the virulence of many bacterial pathogens. Components of the carbohydrate-specific PTS include the general cytoplasmic components enzyme I (EI) and histidine protein (HPr), the sugar-specific cytoplasmic components enzymes IIA (EIIA) and IIB (EIIB), and the sugar-specific membrane-associated multisubunit components enzymes IIC (EIIC) and IID (EIID). Many bacterial genomes also encode a parallel PTS pathway that includes the EI homolog EI(Ntr), the HPr homolog NPr, and the EIIA homolog EIIA(Ntr). This pathway is thought to be nitrogen specific because of the proximity of the genes encoding this pathway to the genes encoding the nitrogen-specific sigma factor sigma(54). We previously reported that phosphorylation of HPr and FPr by EI represses Vibrio cholerae biofilm formation in minimal medium supplemented with glucose or pyruvate. Here we report two additional PTS-based biofilm regulatory pathways that are active in LB broth but not in minimal medium. These pathways involve the glucose-specific enzyme EIIA (EIIA(Glc)) and two nitrogen-specific EIIA homologs, EIIA(Ntr1) and EIIA(Ntr2). The presence of multiple, independent biofilm regulatory circuits in the PTS supports the hypothesis that the PTS and PTS-dependent substrates have a central role in sensing environments suitable for a surface-associated existence.
Figures










Comment in
-
The phosphoenolpyruvate phosphotransferase system: as important for biofilm formation by Vibrio cholerae as it is for metabolism in Escherichia coli.J Bacteriol. 2010 Aug;192(16):4083-5. doi: 10.1128/JB.00641-10. Epub 2010 Jun 18. J Bacteriol. 2010. PMID: 20562301 Free PMC article. No abstract available.
Similar articles
-
Removal of a Membrane Anchor Reveals the Opposing Regulatory Functions of Vibrio cholerae Glucose-Specific Enzyme IIA in Biofilms and the Mammalian Intestine.mBio. 2018 Sep 4;9(5):e00858-18. doi: 10.1128/mBio.00858-18. mBio. 2018. PMID: 30181246 Free PMC article.
-
Glucose-specific enzyme IIA has unique binding partners in the vibrio cholerae biofilm.mBio. 2012 Nov 6;3(6):e00228-12. doi: 10.1128/mBio.00228-12. mBio. 2012. PMID: 23131828 Free PMC article.
-
Cross Talk among Transporters of the Phosphoenolpyruvate-Dependent Phosphotransferase System in Bacillus subtilis.J Bacteriol. 2018 Sep 10;200(19):e00213-18. doi: 10.1128/JB.00213-18. Print 2018 Oct 1. J Bacteriol. 2018. PMID: 30038046 Free PMC article.
-
Carbohydrate Transport by Group Translocation: The Bacterial Phosphoenolpyruvate: Sugar Phosphotransferase System.Subcell Biochem. 2019;92:223-274. doi: 10.1007/978-3-030-18768-2_8. Subcell Biochem. 2019. PMID: 31214989 Review.
-
Transcription regulators controlled by interaction with enzyme IIB components of the phosphoenolpyruvate: sugar phosphotransferase system.Biochim Biophys Acta. 2013 Jul;1834(7):1415-24. doi: 10.1016/j.bbapap.2013.01.004. Epub 2013 Jan 11. Biochim Biophys Acta. 2013. PMID: 23318733 Review.
Cited by
-
In situ proteolysis of the Vibrio cholerae matrix protein RbmA promotes biofilm recruitment.Proc Natl Acad Sci U S A. 2015 Aug 18;112(33):10491-6. doi: 10.1073/pnas.1512424112. Epub 2015 Aug 3. Proc Natl Acad Sci U S A. 2015. PMID: 26240338 Free PMC article.
-
NtrC Adds a New Node to the Complex Regulatory Network of Biofilm Formation and vps Expression in Vibrio cholerae.J Bacteriol. 2018 Jul 10;200(15):e00025-18. doi: 10.1128/JB.00025-18. Print 2018 Aug 1. J Bacteriol. 2018. PMID: 29735756 Free PMC article.
-
Vibrio cholerae genomic diversity within and between patients.Microb Genom. 2017 Dec;3(12):e000142. doi: 10.1099/mgen.0.000142. Microb Genom. 2017. PMID: 29306353 Free PMC article.
-
MtlR negatively regulates mannitol utilization by Vibrio cholerae.Microbiology (Reading). 2017 Dec;163(12):1902-1911. doi: 10.1099/mic.0.000559. Microbiology (Reading). 2017. PMID: 29076803 Free PMC article.
-
Activation of Vibrio cholerae quorum sensing promotes survival of an arthropod host.Nat Microbiol. 2018 Feb;3(2):243-252. doi: 10.1038/s41564-017-0065-7. Epub 2017 Nov 27. Nat Microbiol. 2018. PMID: 29180725 Free PMC article.
References
-
- Barriere, C., M. Veiga-da-Cunha, N. Pons, E. Guedon, S. A. van Hijum, J. Kok, O. P. Kuipers, D. S. Ehrlich, and P. Renault. 2005. Fructose utilization in Lactococcus lactis as a model for low-GC gram-positive bacteria: its regulator, signal, and DNA-binding site. J. Bacteriol. 187:3752-3761. - PMC - PubMed
-
- Berg, T., S. Schild, and J. Reidl. 2007. Regulation of the chitobiose-phosphotransferase system in Vibrio cholerae. Arch. Microbiol. 187:433-439. - PubMed
-
- Beyhan, S., and F. H. Yildiz. 2007. Smooth to rugose phase variation in Vibrio cholerae can be mediated by a single nucleotide change that targets c-di-GMP signalling pathway. Mol. Microbiol. 63:995-1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

