Development of a chip assay and quantitative PCR for detecting microcystin synthetase E gene expression
- PMID: 20400558
- PMCID: PMC2893508
- DOI: 10.1128/AEM.00452-10
Development of a chip assay and quantitative PCR for detecting microcystin synthetase E gene expression
Abstract
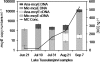
The chip and quantitative real-time PCR (qPCR) assays were optimized to study the expression of microcystin biosynthesis genes (mcy) with RNA samples extracted from cyanobacterial strains and environmental water samples. Both microcystin-producing Anabaena and Microcystis were identified in Lake Tuusulanjärvi samples. Microcystis transcribed the mcyE genes throughout the summer of 2006, while expression by Anabaena became evident later in August and September. Active mcyE gene expression was also detectable when microcystin concentrations were very low. Detection of Anabaena mcyE transcripts by qPCR, as well as certain cyanobacterial 16S rRNAs with the chip assay, showed slightly reduced sensitivity compared with the DNA analyses. In contrast, even groups undetectable or present in low quantities as determined by microscopy could be identified with the chip assay from DNA samples. The methods introduced add to the previously scarce repertoire of applications for mcy expression profiling in environmental samples and enable in situ studies of regulation of microcystin synthesis in response to environmental factors.
Figures



Similar articles
-
Quantitative real-time PCR for determination of microcystin synthetase e copy numbers for microcystis and anabaena in lakes.Appl Environ Microbiol. 2003 Dec;69(12):7289-97. doi: 10.1128/AEM.69.12.7289-7297.2003. Appl Environ Microbiol. 2003. PMID: 14660378 Free PMC article.
-
Molecular methods: chip assay and quantitative real-time PCR: in detecting hepatotoxic cyanobacteria.Methods Mol Biol. 2011;739:73-86. doi: 10.1007/978-1-61779-102-4_7. Methods Mol Biol. 2011. PMID: 21567319
-
Comparison of cyanobacterial microcystin synthetase (mcy) E gene transcript levels, mcy E gene copies, and biomass as indicators of microcystin risk under laboratory and field conditions.Microbiologyopen. 2014 Aug;3(4):411-25. doi: 10.1002/mbo3.173. Epub 2014 May 17. Microbiologyopen. 2014. PMID: 24838591 Free PMC article.
-
Identification of hepatotoxin-producing cyanobacteria by DNA-chip.Environ Microbiol. 2008 Mar;10(3):653-64. doi: 10.1111/j.1462-2920.2007.01488.x. Epub 2008 Jan 7. Environ Microbiol. 2008. PMID: 18190512
-
Genetic contributions to the risk assessment of microcystin in the environment.Toxicol Appl Pharmacol. 2005 Mar 15;203(3):192-200. doi: 10.1016/j.taap.2004.06.008. Toxicol Appl Pharmacol. 2005. PMID: 15737674 Review.
Cited by
-
Characterization and Diversity of Microcystins Produced by Cyanobacteria from the Curonian Lagoon (SE Baltic Sea).Toxins (Basel). 2021 Nov 24;13(12):838. doi: 10.3390/toxins13120838. Toxins (Basel). 2021. PMID: 34941676 Free PMC article.
-
Microbial communities in aerosol generated from cyanobacterial bloom-affected freshwater bodies: an exploratory study in Nakdong River, South Korea.Front Microbiol. 2023 Jul 13;14:1203317. doi: 10.3389/fmicb.2023.1203317. eCollection 2023. Front Microbiol. 2023. PMID: 37520352 Free PMC article.
-
Long-term monitoring reveals carbon-nitrogen metabolism key to microcystin production in eutrophic lakes.Front Microbiol. 2015 May 12;6:456. doi: 10.3389/fmicb.2015.00456. eCollection 2015. Front Microbiol. 2015. PMID: 26029192 Free PMC article.
-
Detection of microcystin-producing cyanobacteria in water samples using loop-mediated isothermal amplification targeting mcyB gene.3 Biotech. 2018 Sep;8(9):378. doi: 10.1007/s13205-018-1402-0. Epub 2018 Aug 17. 3 Biotech. 2018. PMID: 30148028 Free PMC article.
-
Selective enrichment of active bacterial taxa in the Microcystis associated microbiome during colony growth.PeerJ. 2025 Apr 4;13:e19149. doi: 10.7717/peerj.19149. eCollection 2025. PeerJ. 2025. PMID: 40196299 Free PMC article.
References
-
- Bustin, S. A., V. Benes, J. A. Garson, J. Hellemans, J. Huggett, M. Kubista, R. Mueller, T. Nolan, M. W. Pfaffl, G. L. Shipley, J. Vandersompele, and C. T. Wittwer. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55:611-622. - PubMed
-
- Castenholz, R. W. 2001. Oxygenic photosynthetic bacteria, p. 473-599. In D. R. Boone and R. W Castenholz (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer-Verlag, New York, NY.
-
- Castiglioni, B., E. Rizzi, A. Frosini, K. Sivonen, P. Rajaniemi, A. Rantala, M. A. Mugnai, S. Ventura, A. Wilmotte, C. Boutte, S. Grubisic, P. Balthasart, C. Consolandi, R. Bordoni, A. Mezzelani, C. Battaglia, and G. De Bellis. 2004. Development of a universal microarray based on the ligation detection reaction and 16S rRNA gene polymorphism to target diversity of cyanobacteria. Appl. Environ. Microbiol. 70:7161-7172. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

