The myeloid 7/4-antigen defines recently generated inflammatory macrophages and is synonymous with Ly-6B
- PMID: 20400676
- PMCID: PMC2892525
- DOI: 10.1189/jlb.0809548
The myeloid 7/4-antigen defines recently generated inflammatory macrophages and is synonymous with Ly-6B
Erratum in
- J Leukoc Biol. 2011 Nov;90(5):1035
-
Erratum.J Leukoc Biol. 2011 Nov;90(5):1035. doi: 10.1189/jlb.0809548err. J Leukoc Biol. 2011. PMID: 29360164 Free PMC article. No abstract available.
Abstract
This study aimed to identify the inflammation-associated 7/4-antigen, which is highly expressed on neutrophils, inflammatory monocytes, some activated macrophages, as well as on bone marrow myeloid-restricted progenitors. The high expression on inflammatory cells is suggestive of a role in inflammation and makes the 7/4-antigen a potential target for the manipulation of inflammatory cells. Consistent with this, the 7/4-antibody mediates specific depletion of 7/4-expressing neutrophils and monocytes. We have identified the 7/4-antigen as a 25- to 30-kDa GPI-anchored glycoprotein synonymous with the Ly-6B.2 alloantigen. We characterized the expression of Ly-6B during the inflammatory reaction induced by zymosan. During the later stages of an experimental, acute, self-resolving inflammatory response, we found that Ly-6B is differentially expressed on macrophages. Ly-6B-expressing macrophages also express more MHCII, CIITA, CCR2, Ly-6C, and CD62L than the Ly-6B-negative macrophages, which in turn, express more of the resident tissue macrophage marker SIGN-R1 and higher CD11b and F4/80. Ly-6B-expressing macrophages incorporate more BrdU than their Ly-6B-negative contemporaries when fed during the resolution phase of the acute inflammatory response. Thus, Ly-6B expression on mature macrophages defines a subset of recently generated inflammatory macrophages that retain monocytic markers and is hence a surrogate marker of macrophage turnover in inflammatory lesions. The definition of the 7/4:Ly-6B antigen will allow further characterization and specific modulation of Ly-6B-expressing cells in vivo.
Figures
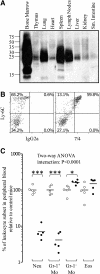
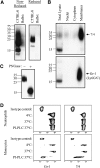

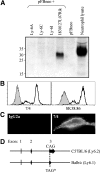


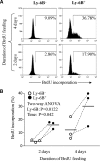
References
-
- Hirsch S, Gordon S. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics. 1983;18:229–239. - PubMed
-
- Gordon S, Keshav S, Stein M. BCG-induced granuloma formation in murine tissues. Immunobiology. 1994;191:369–377. - PubMed
-
- Henderson R B, Hobbs J A, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. - PubMed
-
- Taylor P R, Brown G D, Geldhof A B, Martinez-Pomares L, Gordon S. Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur J Immunol. 2003;33:2090–2097. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

