Renin-angiotensin system activation in congenital hepatic fibrosis in the PCK rat model of autosomal recessive polycystic kidney disease
- PMID: 20400910
- PMCID: PMC4241057
- DOI: 10.1097/MPG.0b013e3181cc80e4
Renin-angiotensin system activation in congenital hepatic fibrosis in the PCK rat model of autosomal recessive polycystic kidney disease
Abstract
Objectives: Congenital hepatic fibrosis (CHF) is an important cause of morbidity and mortality in patients with autosomal recessive polycystic kidney disease (ARPKD). The pathogenesis of CHF remains undefined. Several recent studies suggest that the renin-angiotensin system (RAS) is an important mediator of progressive hepatic fibrosis through activation of profibrotic mediators, such as transforming growth factor-beta (TGF-beta). RAS activation has not previously been studied in patients with CHF or in animal models. The aim of the present study was to characterize RAS expression during the course of CHF in the PCK rat.
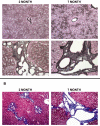
Materials and methods: Studies were conducted in the PCK rat, an orthologous ARPKD/CHF model, and age-matched normal control Sprague-Dawley rats. Expression of the RAS components, renin, angiotensinogen, angiotensin-converting enzyme (ACE), and angiotensin II type 1 receptor (AT1R), as well as the profibrotic mediator TGF-beta, was examined in cystic PCK and control rat livers at 2, 4, and 6 months of age by quantitative real-time polymerase chain reaction (qRT-PCR). Angiotensin II (ANG II) was examined by immunohistochemistry (IHC). Fibrosis was assessed by IHC using reticulin staining and Masson trichrome. Collagen content was determined by hydroxyproline analysis.
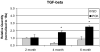
Results: Progressive fibrosis and increased hepatic collagen content occurred in PCK rats with age. In 4- and 6-month-old PCK rat livers, ACE gene expression was markedly increased, 8- and 17-fold, respectively, compared with age-matched control livers. Expression of the other RAS components, renin, angiotensinogen, and AT1R were not significantly different. IHC demonstrated prominent ANG II protein expression in periportal regions in PCK rats. In contrast, no expression was noted in control livers. TGF-beta expression was also increased in PCK rat livers with progressive disease.
Conclusions: The present study demonstrates, for the first time, RAS upregulation in an orthologous rat ARPKD/CHF model. Increases in ACE and ANG II, as well as the downstream target, the profibrotic mediator TGF-beta, suggest that RAS activation may be an important mediator of CHF disease progression. The findings also suggest that treatment with RAS inhibitors, specifically ACE inhibitors or AT1R blockers, could be therapeutic in slowing disease progression in CHF.
Figures


References
-
- Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics. 2003;111:1072–80. - PubMed
-
- Kamath BM, Piccoli DA. Heritable disorders of the bile ducts. Gastroenterol Clin North Am. 2003;32:857–75. vi. - PubMed
-
- Dell KM, Avner ED. GeneClinics: Clinical Genetic Information Resource [database online] Copyright, University of Washington; Seattle: 2001. Autosomal recessive polycystic kidney disease. Available at http://www.geneclinics.org. Initial posting July 2001, updated 2008.
-
- Shneider BL, Magid MS. Liver disease in autosomal recessive polycystic kidney disease. Pediatr Transplant. 2005;9:634–9. - PubMed
-
- Davis ID, Ho M, Hupertz V, Avner ED. Survival of childhood polycystic kidney disease following renal transplantation: the impact of advanced hepatobiliary disease. Pediatr Transplant. 2003;7:364–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

