Glucose-regulated protein precursor (GRP78) and tumor rejection antigen (GP96) are unique to hamster caput epididymal spermatozoa
- PMID: 20400973
- PMCID: PMC3739268
- DOI: 10.1038/aja.2010.19
Glucose-regulated protein precursor (GRP78) and tumor rejection antigen (GP96) are unique to hamster caput epididymal spermatozoa
Abstract
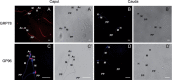
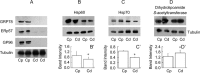
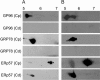
The immotile testicular mammalian spermatozoon gets transformed into a motile spermatozoon during 'epididymal maturation'. During this process, the spermatozoa transit from the caput to the cauda epididymis and undergo a number of distinct morphological, biophysical and biochemical changes, including changes in protein composition and protein modifications, which may be relevant to the acquisition of motility potential. The present proteome-based study of the hamster epididymal spermatozoa of caput and cauda led to the identification of 113 proteins spots using Matrix-assisted laser desorption/ionization tandem mass spectrometry (MALDI-MS/MS) analysis. Comparison of these 113 protein spots indicated that 30 protein spots (corresponding to 20 proteins) were significantly changed in intensity. Five proteins were increased and eleven were decreased in intensity in the cauda epididymal spermatozoa. In addition, two proteins, glucose-regulated protein precursor (GRP78) and tumor rejection antigen (GP96), were unique to the caput epididymal spermatozoa, while one protein, fibrinogen-like protein 1, was unique to cauda epididymal spermatozoa. A few of the five proteins, which increased in intensity, were related to sperm metabolism and ATP production during epididymal maturation. The changes in intensity of a few proteins such as ERp57, GRP78, GP96, Hsp60, Hsp70, and dihydrolipoamide S-acetyltransferase were validated by immunoblotting. The present study provides a global picture of the changes in protein composition occurring during hamster sperm epididymal maturation, besides being the first ever report on the proteome of hamster spermatozoa.
Figures


Similar articles
-
Identification and validation of mouse sperm proteins correlated with epididymal maturation.Proteomics. 2011 Oct;11(20):4047-62. doi: 10.1002/pmic.201100075. Epub 2011 Aug 30. Proteomics. 2011. PMID: 21805633 Free PMC article.
-
Identification and characterization of ERp29 in rat spermatozoa during epididymal transit.Reproduction. 2007 Mar;133(3):575-84. doi: 10.1530/REP-06-0301. Reproduction. 2007. PMID: 17379652
-
Investigation of epididymal sperm maturation in the golden hamster.Int J Androl. 1994 Oct;17(5):256-61. doi: 10.1111/j.1365-2605.1994.tb01251.x. Int J Androl. 1994. PMID: 7698851
-
Role of exosomes in sperm maturation during the transit along the male reproductive tract.Blood Cells Mol Dis. 2005 Jul-Aug;35(1):1-10. doi: 10.1016/j.bcmd.2005.03.005. Blood Cells Mol Dis. 2005. PMID: 15893944 Review.
-
The contribution of proteomics to understanding epididymal maturation of mammalian spermatozoa.Syst Biol Reprod Med. 2012 Aug;58(4):197-210. doi: 10.3109/19396368.2012.663233. Syst Biol Reprod Med. 2012. PMID: 22788532 Review.
Cited by
-
Stallion sperm transcriptome comprises functionally coherent coding and regulatory RNAs as revealed by microarray analysis and RNA-seq.PLoS One. 2013;8(2):e56535. doi: 10.1371/journal.pone.0056535. Epub 2013 Feb 11. PLoS One. 2013. PMID: 23409192 Free PMC article.
-
The human protein disulfide isomerase gene family.Hum Genomics. 2012 Jul 5;6(1):6. doi: 10.1186/1479-7364-6-6. Hum Genomics. 2012. PMID: 23245351 Free PMC article.
-
Molecular changes and signaling events occurring in spermatozoa during epididymal maturation.Andrology. 2017 Mar;5(2):204-218. doi: 10.1111/andr.12320. Andrology. 2017. PMID: 28297559 Free PMC article. Review.
-
Identification and validation of mouse sperm proteins correlated with epididymal maturation.Proteomics. 2011 Oct;11(20):4047-62. doi: 10.1002/pmic.201100075. Epub 2011 Aug 30. Proteomics. 2011. PMID: 21805633 Free PMC article.
-
Epididymal mRNA and miRNA transcriptome analyses reveal important genes and miRNAs related to sperm motility in roosters.Poult Sci. 2022 Jan;101(1):101558. doi: 10.1016/j.psj.2021.101558. Epub 2021 Oct 21. Poult Sci. 2022. PMID: 34844112 Free PMC article.
References
-
- Devi LG, Shivaji S. Computerized analysis of the motility parameters of hamster spermatozoa during maturation. Mol Reprod Dev. 1994;38:94–106. - PubMed
-
- Cooper TG. Interactions between epididymal secretions and spermatozoa. J Reprod Fertil Supp. 1998;53:119–36. - PubMed
-
- McLean DJ. Spermatogonial stem cell transplantation and testicular function. Cell Tissue Res. 2005;322:21–31. - PubMed
-
- Yanagimachi R.Mammalian fertilizationIn: Knobil K, Neill JD, editors. The Physiology of ReproductionNew York, NY: Raven Press; 1994p189–317.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

