TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214
- PMID: 20400975
- PMCID: PMC2889129
- DOI: 10.1038/onc.2010.111
TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214
Abstract
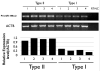
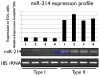
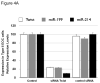
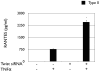
Cancer stem cells are responsible for sustaining the tumor and giving rise to proliferating and progressively differentiating cells. However, the molecular mechanisms regulating the process of cancer stem cell (CSC) differentiation is not clearly understood. Recently, we reported the isolation of the epithelial ovarian cancer (EOC) stem cells (type I/CD44+). In this study, we show that type I/CD44+ cells are characterized by low levels of both miR-199a and miR-214, whereas mature EOC cells (type II/CD44-) have higher levels of miR-199a and miR-214. Moreover, these two micro RNAs (miRNAs) are regulated as a cluster on pri-miR-199a2 within the human Dnm3os gene (GenBank FJ623959). This study identify Twist1 as a regulator of this unique miRNA cluster responsible for the regulation of the IKKbeta/NF-kappaB and PTEN/AKT pathways and its association of ovarian CSC differentiation. Our data suggest that Twist1 may be an important regulator of 'stemness' in EOC cells. The regulation of MIR199A2/214 expression may be used as a potential therapeutic approach in EOC patients.
Figures









Similar articles
-
miR-221/222 promote cancer stem-like cell properties and tumor growth of breast cancer via targeting PTEN and sustained Akt/NF-κB/COX-2 activation.Chem Biol Interact. 2017 Nov 1;277:33-42. doi: 10.1016/j.cbi.2017.08.014. Epub 2017 Aug 24. Chem Biol Interact. 2017. PMID: 28844858
-
MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells.FEBS J. 2012 Jun;279(11):2047-59. doi: 10.1111/j.1742-4658.2012.08589.x. Epub 2012 Apr 24. FEBS J. 2012. PMID: 22498306
-
miR-26b regulates cell proliferation and apoptosis of CD117+CD44+ ovarian cancer stem cells by targeting PTEN.Eur J Histochem. 2021 Feb 4;65(1):3186. doi: 10.4081/ejh.2021.3186. Eur J Histochem. 2021. PMID: 33634678 Free PMC article.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
Roles of miRNAs in regulating ovarian cancer stemness.Biochim Biophys Acta Rev Cancer. 2024 Nov;1879(6):189191. doi: 10.1016/j.bbcan.2024.189191. Epub 2024 Sep 29. Biochim Biophys Acta Rev Cancer. 2024. PMID: 39353485 Review.
Cited by
-
Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis.Reprod Biol Endocrinol. 2015 Jul 22;13:75. doi: 10.1186/s12958-015-0063-7. Reprod Biol Endocrinol. 2015. PMID: 26198055 Free PMC article.
-
Reduction of the ST6 β-galactosamide α-2,6-sialyltransferase 1 (ST6GAL1)-catalyzed sialylation of nectin-like molecule 2/cell adhesion molecule 1 and enhancement of ErbB2/ErbB3 signaling by microRNA-199a.J Biol Chem. 2013 Apr 26;288(17):11845-53. doi: 10.1074/jbc.M112.405993. Epub 2013 Mar 15. J Biol Chem. 2013. PMID: 23504322 Free PMC article.
-
Knockdown of miR-214 promotes apoptosis and inhibits cell proliferation in nasopharyngeal carcinoma.PLoS One. 2014 Jan 21;9(1):e86149. doi: 10.1371/journal.pone.0086149. eCollection 2014. PLoS One. 2014. PMID: 24465927 Free PMC article.
-
MicroRNA profiling reveals that miR-21, miR486 and miR-214 are upregulated and involved in cell survival in Sézary syndrome.Cell Death Dis. 2011 Apr 28;2(4):e151. doi: 10.1038/cddis.2011.32. Cell Death Dis. 2011. PMID: 21525938 Free PMC article.
-
Evidences for a New Role of miR-214 in Chondrogenesis.Sci Rep. 2018 Feb 27;8(1):3704. doi: 10.1038/s41598-018-21735-w. Sci Rep. 2018. PMID: 29487295 Free PMC article.
References
-
- Ahmed N, Thompson EW, Quinn MA. Epithelial-mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: an exception to the norm. J Cell Physiol. 2007;213:581–588. - PubMed
-
- Alison MR, Murphy G, Leedham S. Stem cells and cancer: a deadly mix. Cell Tissue Res. 2008;331:109–124. - PubMed
-
- Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–4662. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

