Identification of Rotavirus VP6-Specific CD4+ T Cell Epitopes in a G1P[8] Human Rotavirus-Infected Rhesus Macaque
- PMID: 20401320
- PMCID: PMC2855136
- DOI: 10.4137/vrt.s563
Identification of Rotavirus VP6-Specific CD4+ T Cell Epitopes in a G1P[8] Human Rotavirus-Infected Rhesus Macaque
Abstract
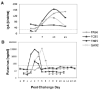
A non-human primate model was used to evaluate its potential for identification of rotavirus viral protein 6 (VP6) CD4+ T cell epitopes. Four juvenile rhesus macaques were inoculated with a mixed inoculum (G1P[8] and G9P[8]) of human rotaviruses. Infection accompanied by G1P[8] shedding was achieved in the two macaques that had no rotavirus immunoglobulin A (IgA) in plasma. To measure the interferon gamma (IFN-γ) and tumor necrosis factor (TNF) anti-viral cytokines produced by peripheral CD4+ cells that recognize VP6 epitopes, whole blood cells from one infected macaque were stimulated in vitro with VP6 peptides. Stimulation with peptide pools derived from the simian rotavirus VP6(161-395) region revealed reactivity of CD4+ T cells with the VP6(281-331) domain. A VP6(301-315) region was identified as the epitope responsible for IFN-γ production while a broader VP6(293-327) domain was linked to TNF production. These results suggest that human rotavirus-infected macaques can be used for identification of additional epitopes and domains to address specific questions related to the development of pediatric vaccines.
Figures

References
-
- Doxiadis GGM, Otting N, de Groot NG, Bontrop RE. Differential evolutionary MHC class II strategied in humans and rhesus macaques: relevance for biomedical studies. Immunol Rev. 2001;183:76–85. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

