Sensing cytoplasmic danger signals by the inflammasome
- PMID: 20401524
- PMCID: PMC3316122
- DOI: 10.1007/s10875-010-9419-0
Sensing cytoplasmic danger signals by the inflammasome
Abstract
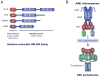
Introduction: The innate immune system depends on molecules collectively known as pattern recognition receptors (PRRs) to survey the extracellular space and the cytoplasm for the presence of dangerous pathogens, pathogen-derived molecules, or even self-derived molecular danger signals, which arise from tissue damage. Absent in melanoma 2 (AIM2) is a newly discovered PRR involved in the sensing of dangerous cytosolic DNA produced by infection with DNA viruses.
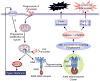
Discussion: Remarkably, recent studies in AIM2-deficient mice showed that AIM2 is uniquely involved in sensing infection with the intracellular bacteria Francisella tularensis and subsequently triggering caspase-1-mediated pro-inflammatory cytokine production and macrophage cell death, which activate other components of the immune system and eliminate the infected macrophages. Here, we provide an overview of our current understanding of the role of AIM2 in innate immunity against F. tularensis in particular, and how infection of macrophages with this pathogen is thought to activate AIM2.
Figures
References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. - PubMed
-
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–25. - PubMed
-
- Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

