An integrated, self-contained microfluidic cassette for isolation, amplification, and detection of nucleic acids
- PMID: 20401537
- PMCID: PMC2924744
- DOI: 10.1007/s10544-010-9423-4
An integrated, self-contained microfluidic cassette for isolation, amplification, and detection of nucleic acids
Abstract
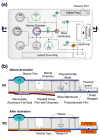
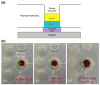
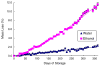
A self-contained, integrated, disposable, sample-to-answer, polycarbonate microfluidic cassette for nucleic acid-based detection of pathogens at the point of care was designed, constructed, and tested. The cassette comprises on-chip sample lysis, nucleic acid isolation, enzymatic amplification (polymerase chain reaction and, when needed, reverse transcription), amplicon labeling, and detection. On-chip pouches and valves facilitate fluid flow control. All the liquids and dry reagents needed for the various reactions are pre-stored in the cassette. The liquid reagents are stored in flexible pouches formed on the chip surface. Dry (RT-)PCR reagents are pre-stored in the thermal cycling, reaction chamber. The process operations include sample introduction; lysis of cells and viruses; solid-phase extraction, concentration, and purification of nucleic acids from the lysate; elution of the nucleic acids into a thermal cycling chamber and mixing with pre-stored (RT-)PCR dry reagents; thermal cycling; and detection. The PCR amplicons are labeled with digoxigenin and biotin and transmitted onto a lateral flow strip, where the target analytes bind to a test line consisting of immobilized avidin-D. The immobilized nucleic acids are labeled with up-converting phosphor (UCP) reporter particles. The operation of the cassette is automatically controlled by an analyzer that provides pouch and valve actuation with electrical motors and heating for the thermal cycling. The functionality of the device is demonstrated by detecting the presence of bacterial B.Cereus, viral armored RNA HIV, and HIV I virus in saliva samples. The cassette and actuator described here can be used to detect other diseases as well as the presence of bacterial and viral pathogens in the water supply and other fluids.
Figures


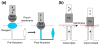





References
-
- Belgrader P, Benett W, Hadley D, Richards J, Stratton P, Mariella R, Jr, Milanovich F. Science. 1999;284:449–450. - PubMed
-
- Belgrader P, Young S, Yuan B, Primeau M, Christel LA, Pourahmadi F, Northrup MA. Anal Chem. 2001;73:286–289. - PubMed
-
- Blatt JM, Allen MP, Baddam S, Chase CL, Dasu BN, Dickens DM, Hardt RTHSJ, Hsu Y, Kitazawa CT, Li S, Mangan WM, Patel PJ, Pfeiffer JW, Quiwa NB, Scratch MA, Widunas JT. Clin Chem. Vol. 44. Washington, D. C.: 1998. pp. 2051–2052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

