Gold nanoparticles for biology and medicine
- PMID: 20401880
- PMCID: PMC3930332
- DOI: 10.1002/anie.200904359
Gold nanoparticles for biology and medicine
Abstract
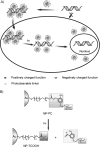
Gold colloids have fascinated scientists for over a century and are now heavily utilized in chemistry, biology, engineering, and medicine. Today these materials can be synthesized reproducibly, modified with seemingly limitless chemical functional groups, and, in certain cases, characterized with atomic-level precision. This Review highlights recent advances in the synthesis, bioconjugation, and cellular uses of gold nanoconjugates. There are now many examples of highly sensitive and selective assays based upon gold nanoconjugates. In recent years, focus has turned to therapeutic possibilities for such materials. Structures which behave as gene-regulating agents, drug carriers, imaging agents, and photoresponsive therapeutics have been developed and studied in the context of cells and many debilitating diseases. These structures are not simply chosen as alternatives to molecule-based systems, but rather for their new physical and chemical properties, which confer substantive advantages in cellular and medical applications.
Figures





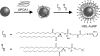
References
-
- Hayat MA. Colloidal gold: principles, methods, and applications. Academic Press; San Diego: 1989.
-
- Daniel MC, Astruc D. Chem Rev. 2004;104:293. - PubMed
-
- Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. Nature. 1996;382:607. - PubMed
-
- Park SY, Lytton-Jean AKR, Lee B, Weigand S, Schatz GC, Mirkin CA. Nature. 2008;451:553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

