X-ray structures of isopentenyl phosphate kinase
- PMID: 20402538
- PMCID: PMC2879073
- DOI: 10.1021/cb100032g
X-ray structures of isopentenyl phosphate kinase
Abstract
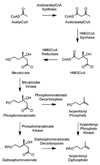
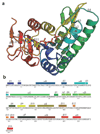
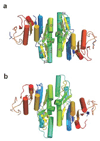
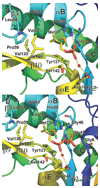
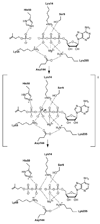
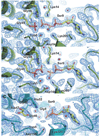
Isoprenoid compounds are ubiquitous in nature, participating in important biological phenomena such as signal transduction, aerobic cellular respiration, photosynthesis, insect communication, and many others. They are derived from the 5-carbon isoprenoid substrates isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP). In Archaea and Eukarya, these building blocks are synthesized via the mevalonate pathway. However, the genes required to convert mevalonate phosphate (MP) to IPP are missing in several species of Archaea. An enzyme with isopentenyl phosphate kinase (IPK) activity was recently discovered in Methanocaldococcus jannaschii (MJ), suggesting a departure from the classical sequence of converting MP to IPP. We have determined the high-resolution crystal structures of isopentenyl phosphate kinases in complex with both substrates and products from Thermoplasma acidophilum (THA), as well as the IPK from Methanothermobacter thermautotrophicus (MTH), by means of single-wavelength anomalous diffraction (SAD) and molecular replacement. A histidine residue (His50) in THA IPK makes a hydrogen bond with the terminal phosphates of IP and IPP, poising these molecules for phosphoryl transfer through an in-line geometry. Moreover, a lysine residue (Lys14) makes hydrogen bonds with nonbridging oxygen atoms at P(alpha) and P(gamma) and with the P(beta)-P(gamma) bridging oxygen atom in ATP. These interactions suggest a transition-state-stabilizing role for this residue. Lys14 is a part of a newly discovered "lysine triangle" catalytic motif in IPKs that also includes Lys5 and Lys205. Moreover, His50, Lys5, Lys14, and Lys205 are conserved in all IPKs and can therefore serve as fingerprints for identifying new homologues.
Figures
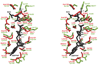
Similar articles
-
Characterization of thermophilic archaeal isopentenyl phosphate kinases.Biochemistry. 2010 Jan 12;49(1):207-17. doi: 10.1021/bi9017957. Biochemistry. 2010. PMID: 19928876 Free PMC article.
-
Mutation of archaeal isopentenyl phosphate kinase highlights mechanism and guides phosphorylation of additional isoprenoid monophosphates.ACS Chem Biol. 2010 Jun 18;5(6):589-601. doi: 10.1021/cb1000313. ACS Chem Biol. 2010. PMID: 20392112 Free PMC article.
-
The Streptomyces-produced antibiotic fosfomycin is a promiscuous substrate for archaeal isopentenyl phosphate kinase.Biochemistry. 2012 Jan 31;51(4):917-25. doi: 10.1021/bi201662k. Epub 2012 Jan 11. Biochemistry. 2012. PMID: 22148590 Free PMC article.
-
Development of isopentenyl phosphate kinases and their application in terpenoid biosynthesis.Biotechnol Adv. 2023 May-Jun;64:108124. doi: 10.1016/j.biotechadv.2023.108124. Epub 2023 Mar 1. Biotechnol Adv. 2023. PMID: 36863457 Review.
-
Current development in isoprenoid precursor biosynthesis and regulation.Curr Opin Chem Biol. 2013 Aug;17(4):571-9. doi: 10.1016/j.cbpa.2013.06.020. Epub 2013 Jul 24. Curr Opin Chem Biol. 2013. PMID: 23891475 Free PMC article. Review.
Cited by
-
Structural and biochemical insights into the mechanism of fosfomycin phosphorylation by fosfomycin resistance kinase FomA.Biochemistry. 2011 Aug 16;50(32):6909-19. doi: 10.1021/bi2004334. Epub 2011 Jul 18. Biochemistry. 2011. PMID: 21728358 Free PMC article.
-
In silico docking and molecular dynamics for the discovery of inhibitors of enteric methane production in ruminants - A review.Anim Biosci. 2025 Jan;38(1):1-18. doi: 10.5713/ab.24.0291. Epub 2024 Aug 26. Anim Biosci. 2025. PMID: 39210806 Free PMC article.
-
Methylerythritol phosphate pathway of isoprenoid biosynthesis.Annu Rev Biochem. 2013;82:497-530. doi: 10.1146/annurev-biochem-052010-100934. Annu Rev Biochem. 2013. PMID: 23746261 Free PMC article. Review.
-
Improving the catalytic activity of isopentenyl phosphate kinase through protein coevolution analysis.Sci Rep. 2016 Apr 7;6:24117. doi: 10.1038/srep24117. Sci Rep. 2016. PMID: 27052337 Free PMC article.
-
Ternary complexes of isopentenyl phosphate kinase from Thermococcus paralvinellae reveal molecular determinants of non-natural substrate specificity.Proteins. 2024 Jul;92(7):808-818. doi: 10.1002/prot.26674. Epub 2024 Feb 9. Proteins. 2024. PMID: 38333996 Free PMC article.
References
-
- Reiling KK, Yoshikuni Y, Martin VJJ, Newman J, Bohlmann J, Keasling JD. Mono and diterpene production in Escherichia coli. Biotechnol. Bioeng. 2004;87:200–212. - PubMed
-
- Davis EM, Croteau R. Cyclization enzyme in the biosynthesis of monoterpenes, sesquiterpenes, and di-terpenes. Top. Curr. Chem. 2000;209:53–95.
-
- Liang P, Ko T, Wang AH. Structure, mechanism and function of prenyltransferases. Eur. J. Biochem. 2002;269:3339–3354. - PubMed
-
- Sacchetini JC, Poulter CD. Creating isoprenoid diversity. Science. 1997;277:1788–1789. - PubMed
-
- Ogura K, Koyama T. Enzymatic aspects of isoprenoid chain elongation. Chem. Rev. 1998;98:1263–1276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

