Suppression of farnesyltransferase activity in acute myeloid leukemia and myelodysplastic syndrome: current understanding and recommended use of tipifarnib
- PMID: 20402600
- PMCID: PMC3252817
- DOI: 10.1517/13543781003801076
Suppression of farnesyltransferase activity in acute myeloid leukemia and myelodysplastic syndrome: current understanding and recommended use of tipifarnib
Abstract
Importance of the field: Acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) incidence in the United States increases with age. Given the progressive ageing of the general population, incidence of these diseases is likely to continue to rise in the future. There is an acute need for therapeutic developments because of the poor prognosis of these diseases. Since the knowledge of molecular genetics in AML and MDS has expanded recently, targeted therapeutics should offer an exciting new frontier for advancement. Of all the targeted inhibitors developed, tipifarnib represents one of the few compounds with some activity as a single agent.
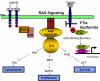
Areas covered in this review: Described in this review are the molecular targets of tipifarnib, safety and tolerability of the drug, chemistry, and clinical efficacy in AML.
What the reader will gain: The reader will gain a thorough understanding of tipifarnib as it relates to the current and future use of the drug in AML.
Take home message: The future of tipifarnib, along with other molecularly-targeted drugs, lies in achieving a better understanding of leukemia biology and harnessing the activity of this agent using predictive biomarkers for improved patient selection.
Figures
References
-
- Agrawal AG, Somani RR. Farnesyltransferase inhibitor as anticancer agent. Mini Rev Med Chem. 2009;9(6):638–52. - PubMed
-
Review article that describes the development of FTIs for the treatment of cancer.
-
- Alsina M, Fonseca R, et al. Farnesyltransferase inhibitor tipifarnib is well tolerated, induces stabilization of disease, and inhibits farnesylation and oncogenic/tumor survival pathways in patients with advanced multiple myeloma. Blood. 2004;103(9):3271–7. - PubMed
-
Manuscript describes a phase II trial of tipifarnib for the treatment of patients with multiple myeloma (MM).
-
- Auewarakul CU, Lauhakirti D, et al. Frequency of RAS gene mutation and its cooperative genetic events in Southeast Asian adult acute myeloid leukemia. Eur J Haematol. 2006;77(1):51–6. - PubMed
-
Report that describes the frequency of Ras mutations in 239 Thai de novo adult AML patients using polymerase chain reaction-single-strand conformational polymorphism analysis.
-
- Bacher U, Haferlach T, et al. Implications of NRAS mutations in AML: a study of 2502 patients. Blood. 2006;107(10):3847–53. - PubMed
-
In this manuscript, the authors analyzed 2502 patients with acute myeloid leukemia at diagnosis for NRAS mutations around the hot spots at codons 12, 13, and 61 and correlated the results to cytomorphology, cytogenetics, other molecular markers, and prognostic relevance of these mutations.
-
- Basso AD, Kirschmeier P, et al. Lipid posttranslational modifications. Farnesyl transferase inhibitors. J Lipid Res. 2006;47(1):15–31. - PubMed
-
Article describing the role and proteins that undergo prenylation and the role of this modication on the transforming activity of Ras.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
