Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic
- PMID: 20402623
- PMCID: PMC2885142
- DOI: 10.1615/critrevtherdrugcarriersyst.v26.i6.10
Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic
Abstract
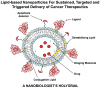
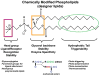
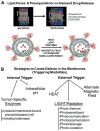
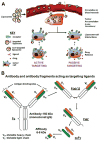
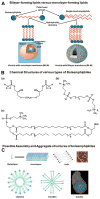
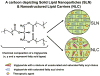
In recent years, various nanotechnology platforms in the area of medical biology, including both diagnostics and therapy, have gained remarkable attention. Moreover, research and development of engineered multifunctional nanoparticles as pharmaceutical drug carriers have spurred exponential growth in applications to medicine in the last decade. Design principles of these nanoparticles, including nanoemulsions, dendrimers, nano-gold, liposomes, drug-carrier conjugates, antibody-drug complexes, and magnetic nanoparticles, are primarily based on unique assemblies of synthetic, natural, or biological components, including but not limited to synthetic polymers, metal ions, oils, and lipids as their building blocks. However, the potential success of these particles in the clinic relies on consideration of important parameters such as nanoparticle fabrication strategies, their physical properties, drug loading efficiencies, drug release potential, and, most importantly, minimum toxicity of the carrier itself. Among these, lipid-based nanoparticles bear the advantage of being the least toxic for in vivo applications, and significant progress has been made in the area of DNA/RNA and drug delivery using lipid-based nanoassemblies. In this review, we will primarily focus on the recent advances and updates on lipid-based nanoparticles for their projected applications in drug delivery. We begin with a review of current activities in the field of liposomes (the so-called honorary nanoparticles), and challenging issues of targeting and triggering will be discussed in detail. We will further describe nanoparticles derived from a novel class of amphipathic lipids called bolaamphiphiles with unique lipid assembly features that have been recently examined as drug/DNA delivery vehicles. Finally, an overview of an emerging novel class of particles (based on lipid components other than phospholipids), solid lipid nanoparticles and nanostructured lipid carriers will be presented. We conclude with a few examples of clinically successful formulations of currently available lipid-based nanoparticles.
Figures


References
-
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine. therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–9. - PubMed
-
- Alaouie AM, Sofou S. Liposomes with triggered content release for cancer therapy. J Biomed Nanotech. 2008;4(3):234–4.
-
- Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–22. - PubMed
-
- Alonso MJ. Nanomedicines for overcoming biological barriers. Biomed Pharmacother. 2004;58(3):168–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

