Epstein-Barr virus neutralizing and early antigen antibodies in multiple sclerosis
- PMID: 20402753
- PMCID: PMC2924906
- DOI: 10.1111/j.1468-1331.2010.03005.x
Epstein-Barr virus neutralizing and early antigen antibodies in multiple sclerosis
Abstract
Background: Our objective was to determine whether antibodies against the Epstein-Barr virus (EBV) nuclear antigen-1 (EBNA-1), early antigen (EA), and EBV neutralizing antibodies (NeutAb) are altered in multiple sclerosis (MS).
Methods: We measured EBNA-1 IgG, EA IgG, and EA IgA using quantitative ELISA. We measured NeutAb using a quantitative competitive ELISA. We studied 80 patients with MS, 80 matched controls, and 19 patients with MS with samples collected both whilst stable and in relapse.
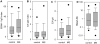
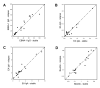
Results: Epstein-Barr virus nuclear antigen-1 IgG and EA IgA were increased in MS compared to controls. The EBNA-1 index value was 23.3 ± 18.3 in the patients with MS (mean ± SD) and 16.3 ± 17.4 in the controls (P = 0.007, paired t-test). EA IgA had a median value of 1.964 in the patients with MS and 1.248 in the controls (P = 0.029, Wilcoxon signed rank test). EA IgG and NeutAb were not significantly different. None of the antibody levels were altered in relapse. The correlation between concentrations of different antibodies was minimal.
Conclusions: IgG antibodies to EBNA-1 are significantly increased in MS. IgA antibodies against EBV EA are also increased. The EBV neutralizing antibody response is similar in MS and controls.
© 2010 The Author(s). European Journal of Neurology © 2010 EFNS.
Figures

Similar articles
-
Epstein-Barr virus and disease activity in multiple sclerosis.J Neurol Neurosurg Psychiatry. 2005 Oct;76(10):1377-81. doi: 10.1136/jnnp.2004.048504. J Neurol Neurosurg Psychiatry. 2005. PMID: 16170080 Free PMC article.
-
Humoral immune response to EBV in multiple sclerosis is associated with disease activity on MRI.Neurology. 2009 Jul 7;73(1):32-8. doi: 10.1212/WNL.0b013e3181aa29fe. Epub 2009 May 20. Neurology. 2009. PMID: 19458321 Free PMC article.
-
Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study.JAMA. 2001 Dec 26;286(24):3083-8. doi: 10.1001/jama.286.24.3083. JAMA. 2001. PMID: 11754673
-
Serological Evidence for the Association Between Epstein-Barr Virus Infection and Sjögren's Syndrome.Front Immunol. 2020 Oct 30;11:590444. doi: 10.3389/fimmu.2020.590444. eCollection 2020. Front Immunol. 2020. PMID: 33193425 Free PMC article.
-
Low intrathecal antibody production despite high seroprevalence of Epstein-Barr virus in multiple sclerosis: a review of the literature.J Neurol. 2018 Feb;265(2):239-252. doi: 10.1007/s00415-017-8656-z. Epub 2017 Nov 2. J Neurol. 2018. PMID: 29098417 Review.
Cited by
-
Antibodies to Human Herpesviruses in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients.Front Immunol. 2019 Aug 14;10:1946. doi: 10.3389/fimmu.2019.01946. eCollection 2019. Front Immunol. 2019. PMID: 31475007 Free PMC article.
-
Epstein-Barr virus neutralizing antibody levels and risk of multiple sclerosis.Mult Scler. 2012 Aug;18(8):1185-7. doi: 10.1177/1352458511433920. Epub 2012 Jan 30. Mult Scler. 2012. PMID: 22291034 Free PMC article.
-
Antibodies specific for Epstein-Barr virus nuclear antigen-1 cross-react with human heterogeneous nuclear ribonucleoprotein L.Mol Immunol. 2016 Jan;69:7-12. doi: 10.1016/j.molimm.2015.11.007. Epub 2015 Nov 28. Mol Immunol. 2016. PMID: 26637929 Free PMC article.
-
Epstein-Barr virus and multiple sclerosis: potential opportunities for immunotherapy.Clin Transl Immunology. 2014 Oct 31;3(10):e27. doi: 10.1038/cti.2014.25. eCollection 2014 Oct. Clin Transl Immunology. 2014. PMID: 25505955 Free PMC article. Review.
-
Discrepancy of Serological and Molecular Patterns of Circulating Epstein-Barr Virus Reactivation in Primary Sjögren's Syndrome.Front Immunol. 2019 May 29;10:1153. doi: 10.3389/fimmu.2019.01153. eCollection 2019. Front Immunol. 2019. PMID: 31191532 Free PMC article.
References
-
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann Neurol. 2007;61:288–299. - PubMed
-
- Lunemann JD, Munz C. EBV in MS: guilty by association? Trends Immunol. 2009;30:243–248. - PubMed
-
- Ascherio A, Munch M. Epstein-Barr virus and multiple sclerosis. Epidemiology. 2000;11:220–224. - PubMed
-
- Pohl D, Krone B, Rostasy K, et al. High seroprevalence of Epstein-Barr virus in children with multiple sclerosis. Neurology. 2006;67:2063–2065. - PubMed
-
- Banwell B, Krupp L, Kennedy J, et al. Clinical features and viral serologies in children with multiple sclerosis: a multinational observational study. Lancet Neurol. 2007;6:773–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

