Induction of neutralizing antibodies in mice immunized with an amino-terminal polypeptide of Streptococcus mutans P1 protein produced by a recombinant Bacillus subtilis strain
- PMID: 20402772
- PMCID: PMC4369793
- DOI: 10.1111/j.1574-695X.2010.00669.x
Induction of neutralizing antibodies in mice immunized with an amino-terminal polypeptide of Streptococcus mutans P1 protein produced by a recombinant Bacillus subtilis strain
Abstract
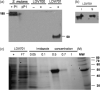
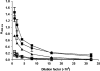
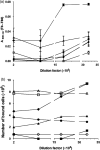
The oral pathogen Streptococcus mutans expresses a surface protein, P1, which interacts with the salivary pellicle on the tooth surface or with fluid-phase saliva, resulting in bacterial adhesion or aggregation, respectively. P1 is a target of protective immunity. Its N-terminal region has been associated with adhesion and aggregation functions and contains epitopes recognized by efficacious antibodies. In this study, we used Bacillus subtilis, a gram-positive expression host, to produce a recombinant N-terminal polypeptide of P1 (P1(39-512)) derived from the S. mutans strain UA159. Purified P1(39-512) reacted with an anti-full-length P1 antiserum as well as one raised against intact S. mutans cells, indicating preserved antigenicity. Immunization of mice with soluble and heat-denatured P1(39-512) induced antibodies that reacted specifically with native P1 on the surface of S. mutans cells. The anti-P1(39-512) antiserum was as effective at blocking saliva-mediated aggregation of S. mutans cells and better at blocking bacterial adhesion to saliva-coated plastic surfaces compared with the anti-full-length P1 antiserum. In addition, adsorption of the anti-P1 antiserum with P1(39-512) eliminated its ability to block the adhesion of S. mutans cells to abiotic surfaces. The present results indicate that P1(39-512), expressed and purified from a recombinant B. subtilis strain, maintains important immunological features of the native protein and represents an additional tool for the development of anticaries vaccines.
Figures

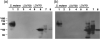

Similar articles
-
Immunogenicity and in vitro and in vivo protective effects of antibodies targeting a recombinant form of the Streptococcus mutans P1 surface protein.Infect Immun. 2014 Dec;82(12):4978-88. doi: 10.1128/IAI.02074-14. Epub 2014 Sep 15. Infect Immun. 2014. PMID: 25225243 Free PMC article.
-
Redirecting the humoral immune response against Streptococcus mutans antigen P1 with monoclonal antibodies.Infect Immun. 2004 Dec;72(12):6951-60. doi: 10.1128/IAI.72.12.6951-6960.2004. Infect Immun. 2004. PMID: 15557617 Free PMC article.
-
Beneficial immunomodulation by Streptococcus mutans anti-P1 monoclonal antibodies is Fc independent and correlates with increased exposure of a relevant target epitope.J Immunol. 2009 Oct 1;183(7):4628-38. doi: 10.4049/jimmunol.0803300. Epub 2009 Sep 14. J Immunol. 2009. PMID: 19752237 Free PMC article.
-
Secretory immunity in defense against cariogenic mutans streptococci.Caries Res. 1999;33(1):4-15. doi: 10.1159/000016490. Caries Res. 1999. PMID: 9831775 Review.
-
Molecular, immunological and functional characterization of the major surface adhesin of Streptococcus mutans.Adv Exp Med Biol. 1992;327:229-41. doi: 10.1007/978-1-4615-3410-5_25. Adv Exp Med Biol. 1992. PMID: 1295342 Review.
Cited by
-
Gut adhesive Bacillus subtilis spores as a platform for mucosal delivery of antigens.Infect Immun. 2014 Apr;82(4):1414-23. doi: 10.1128/IAI.01255-13. Epub 2014 Jan 13. Infect Immun. 2014. PMID: 24421038 Free PMC article.
-
Immunogenicity and in vitro and in vivo protective effects of antibodies targeting a recombinant form of the Streptococcus mutans P1 surface protein.Infect Immun. 2014 Dec;82(12):4978-88. doi: 10.1128/IAI.02074-14. Epub 2014 Sep 15. Infect Immun. 2014. PMID: 25225243 Free PMC article.
-
Bacterial Spore-Based Delivery System: 20 Years of a Versatile Approach for Innovative Vaccines.Biomolecules. 2023 Jun 6;13(6):947. doi: 10.3390/biom13060947. Biomolecules. 2023. PMID: 37371527 Free PMC article. Review.
-
Streptococcus mutans glutamate binding protein (GlnH) as antigen target for a mucosal anti-caries vaccine.Braz J Microbiol. 2022 Dec;53(4):1941-1949. doi: 10.1007/s42770-022-00823-0. Epub 2022 Sep 13. Braz J Microbiol. 2022. PMID: 36098933 Free PMC article.
-
Bacillus subtilis spores as vaccine adjuvants: further insights into the mechanisms of action.PLoS One. 2014 Jan 27;9(1):e87454. doi: 10.1371/journal.pone.0087454. eCollection 2014. PLoS One. 2014. PMID: 24475289 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
