Mitochondrial protection after traumatic brain injury by scavenging lipid peroxyl radicals
- PMID: 20403083
- PMCID: PMC3526891
- DOI: 10.1111/j.1471-4159.2010.06749.x
Mitochondrial protection after traumatic brain injury by scavenging lipid peroxyl radicals
Abstract
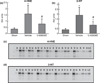
Mitochondrial dysfunction after traumatic brain injury (TBI) is manifested by increased levels of oxidative damage, loss of respiratory functions and diminished ability to buffer cytosolic calcium. This study investigated the detrimental effects of lipid peroxyl radicals (LOO(*)) and lipid peroxidation (LP) in brain mitochondria after TBI by examining the protective effects of U-83836E, a potent and selective scavenger of LOO(*) radicals. Male CF1 mice were subjected to severe controlled cortical impact TBI (CCI-TBI) and treated with either vehicle or U-83836E initiated i.v. at 15 min post-injury. Calcium (Ca(++)) buffering capacity and respiratory function were measured in isolated cortical mitochondrial samples taken from the ipsilateral hemisphere at 3 and 12 h post-TBI, respectively. In vehicle-treated injured mice, the cortical mitochondrial Ca(++) buffering capacity was reduced by 60% at 3 h post-injury (p < 0.001) and the respiratory control ratio was decreased by 27% at 12 h post-TBI, relative to sham, non-injured mice. U-83836E treatment significantly (p < 0.05) preserved Ca(++) buffering capacity and attenuated the reduction in respiratory control ratio values. Consistent with the functional effects of U-83836E being as a result of an attenuation of mitochondrial oxidative damage, the compound significantly (p < 0.001) reduced LP-generated 4-hydroxynonenal levels in both cortical homogenates and mitochondria at both 3 and 12 h post-TBI. Unexpectedly, U-83836E also reduced peroxynitrite-generated 3-nitrotyrosine in parallel with the reduction in 4-hydroxynonenal. The results demonstrate that LOO(*) radicals contribute to secondary brain mitochondrial dysfunction after TBI by propagating LP and protein nitrative damage in cellular and mitochondrial membranes.
Figures




References
-
- Alvarez B, Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25:295–311. - PubMed
-
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, ugly. Am. J. Physiol. 1996;271:C1424–C1437. - PubMed
-
- Brown MR, Sullivan PG, Dorenbos KA, Modafferi EA, Geddes JW, Steward O. Nitrogen disruption of synaptoneurosomes: an alternative method to isolate brain mitochondria. J. Neurosci. Methods. 2004;137:299–303. - PubMed
-
- Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J. Neurochem. 2002;80:207–218. - PubMed
-
- Budd SL, Nicholls DG. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J. Neurochem. 1996;66:403–411. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

