PECAM-1 functions as a negative regulator of laminin-induced platelet activation
- PMID: 20403098
- PMCID: PMC2909358
- DOI: 10.1111/j.1538-7836.2010.03883.x
PECAM-1 functions as a negative regulator of laminin-induced platelet activation
Abstract
Summary background: Interaction of resting platelets with exposed components of the subendothelial matrix is an important early activating event that takes place at sites of vascular injury. Platelet responses to collagen are mediated by integrin alpha(2)beta(1) and the glycoprotein (GP)VI-Fc receptor (FcR) gamma-chain complex, whereas platelet activation by laminin is mediated by the related integrin, alpha(6)beta(1), and similarly requires signaling through GPVI-FcR gamma-chain.
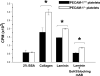
Objective: Because the cell adhesion and signaling receptor PECAM-1 has previously been shown to dampen collagen-induced platelet activation, we sought to determine whether PECAM-1 might similarly regulate platelet activation by laminin.
Methods/results: We found that PECAM-1 became tyrosine phosphorylated on its cytoplasmic immunoreceptor tyrosine-based inhibitory motifs following adhesion of either human or murine platelets to immobilized laminin. Whereas the presence or absence of PECAM-1 had no effect on either the rate or extent of platelet adhesion or spreading on laminin, PECAM-1 inhibited laminin-induced phosphorylation of GPVI-FcR gamma-chain immunoreceptor tyrosine-based activation motifs (ITAMs) and activation of its downstream effector, Syk kinase, and suppressed granule secretion.
Conclusions: Taken together, these data are consistent with previous findings in platelets and other blood and vascular cells that PECAM-1 functions by modulating ITAM-mediated signaling pathways that amplify cellular activation.
Figures






References
-
- Scheele S, Nystrom A, Durbeej M, Talts JF, Ekblom M, Ekblom P. Laminin isoforms in development and disease. J Mol Med. 2007;85:825–36. - PubMed
-
- Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109:5087–95. - PubMed
-
- Newman PJ, Newman DK. Platelets and the Vessel Wall. 7th Edition 2008. pp. 1378–98.
-
- Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin αIIb β3 signaling in platelets. J Thromb Haemost. 2005;3:1752–62. - PubMed
-
- Newton-Nash DK, Newman PJ. A new role for PECAM-1 (CD31): Inhibition of TCR-mediated signal transduction. J Immunol. 1999;163:682–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

