Non-redundant functions of two proline dehydrogenase isoforms in Arabidopsis
- PMID: 20403182
- PMCID: PMC3095344
- DOI: 10.1186/1471-2229-10-70
Non-redundant functions of two proline dehydrogenase isoforms in Arabidopsis
Abstract
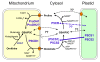
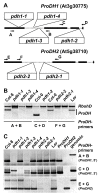
Background: Proline (Pro) accumulation is a widespread response of prokaryotic and eukaryotic cells subjected to osmotic stress or dehydration. When the cells are released from stress, Pro is degraded to glutamate by Pro-dehydrogenase (ProDH) and Pyrroline-5-carboxylate dehydrogenase (P5CDH), which are both mitochondrial enzymes in eukaryotes. While P5CDH is a single copy gene in Arabidopsis, two ProDH genes have been identified in the genome. Until now, only ProDH1 (At3g30775) had been functionally characterised.
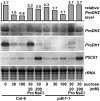
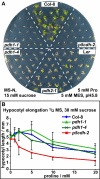
Results: We demonstrate vasculature specific expression of the Arabidopsis ProDH2 gene (At5g38710) as well as enzymatic activity and mitochondrial localisation of the encoded protein. Expression levels of ProDH2 are generally low, but increased in senescent leaves and in the abscission zone of floral organs. While sucrose represses ProDH2 expression, Pro and NaCl were identified as inducers. Endogenous ProDH2 expression was not able to overcome Pro sensitivity of ProDH1 mutants, but overexpression of a GFP-tagged form of ProDH2 enabled the utilisation of Pro as single nitrogen source for growth. Amongst two intronic insertion mutants, one was identified as a null allele, whereas the other still produced native ProDH2 transcripts.
Conclusions: Arabidopsis possesses two functional ProDHs, which have non-redundant, although partially overlapping physiological functions. The two ProDH isoforms differ with respect to spatial, developmental and environmental regulation of expression. While ProDH1 appears to be the dominant isoform under most conditions and in most tissues, ProDH2 was specifically upregulated during salt stress, when ProDH1 was repressed. The characterisation of ProDH2 as a functional gene requires a careful re-analysis of mutants with a deletion of ProDH1, which were so far considered to be devoid of ProDH activity. We hypothesise that ProDH2 plays an important role in Pro homeostasis in the vasculature, especially under stress conditions that promote Pro accumulation.
Figures


Similar articles
-
Differential control and function of Arabidopsis ProDH1 and ProDH2 genes on infection with biotrophic and necrotrophic pathogens.Mol Plant Pathol. 2017 Oct;18(8):1164-1174. doi: 10.1111/mpp.12470. Epub 2016 Oct 17. Mol Plant Pathol. 2017. PMID: 27526663 Free PMC article.
-
Unraveling delta1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes.J Biol Chem. 2009 Sep 25;284(39):26482-92. doi: 10.1074/jbc.M109.009340. Epub 2009 Jul 27. J Biol Chem. 2009. PMID: 19635803 Free PMC article.
-
The N-terminal domain of Arabidopsis proline dehydrogenase affects enzymatic activity and protein oligomerization.Plant Physiol Biochem. 2020 Sep;154:268-276. doi: 10.1016/j.plaphy.2020.04.019. Epub 2020 Jun 13. Plant Physiol Biochem. 2020. PMID: 32574985
-
Regulation of levels of proline as an osmolyte in plants under water stress.Plant Cell Physiol. 1997 Oct;38(10):1095-102. doi: 10.1093/oxfordjournals.pcp.a029093. Plant Cell Physiol. 1997. PMID: 9399433 Review.
-
Proline dehydrogenase: a key enzyme in controlling cellular homeostasis.Front Biosci (Landmark Ed). 2012 Jan 1;17(2):607-20. doi: 10.2741/3947. Front Biosci (Landmark Ed). 2012. PMID: 22201764 Review.
Cited by
-
OsProDH Negatively Regulates Thermotolerance in Rice by Modulating Proline Metabolism and Reactive Oxygen Species Scavenging.Rice (N Y). 2020 Aug 26;13(1):61. doi: 10.1186/s12284-020-00422-3. Rice (N Y). 2020. PMID: 32845426 Free PMC article.
-
Proline oxidation fuels mitochondrial respiration during dark-induced leaf senescence in Arabidopsis thaliana.J Exp Bot. 2019 Nov 18;70(21):6203-6214. doi: 10.1093/jxb/erz351. J Exp Bot. 2019. PMID: 31504781 Free PMC article.
-
Structural and Biochemical Characterization of Aldehyde Dehydrogenase 12, the Last Enzyme of Proline Catabolism in Plants.J Mol Biol. 2019 Feb 1;431(3):576-592. doi: 10.1016/j.jmb.2018.12.010. Epub 2018 Dec 21. J Mol Biol. 2019. PMID: 30580036 Free PMC article.
-
Arabidopsis ACA7, encoding a putative auto-regulated Ca(2+)-ATPase, is required for normal pollen development.Plant Cell Rep. 2012 Apr;31(4):651-9. doi: 10.1007/s00299-011-1182-z. Epub 2011 Nov 2. Plant Cell Rep. 2012. PMID: 22044965
-
Leaf developmental stage modulates metabolite accumulation and photosynthesis contributing to acclimation of Arabidopsis thaliana to water deficit.J Plant Res. 2014 Jul;127(4):481-9. doi: 10.1007/s10265-014-0635-1. Epub 2014 May 22. J Plant Res. 2014. PMID: 24848774
References
-
- Nanjo T, Kobayashi M, Yoshiba Y, Sanada Y, Wada K, Tsukaya H, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J. 1999;18(2):185–193. doi: 10.1046/j.1365-313X.1999.00438.x. - DOI - PubMed
-
- Szekely G, Abraham E, Cseplo A, Rigo G, Zsigmond L, Csiszar J, Ayaydin F, Strizhov N, Jasik J, Schmelzer E. et al.Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant Journal. 2008;53(1):11–28. doi: 10.1111/j.1365-313X.2007.03318.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

