Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations
- PMID: 20403324
- PMCID: PMC2876819
- DOI: 10.1016/j.cell.2010.02.026
Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations
Abstract
Mitochondria are highly mobile and dynamic organelles that continually fuse and divide. These processes allow mitochondria to exchange contents, including mitochondrial DNA (mtDNA). Here we examine the functions of mitochondrial fusion in differentiated skeletal muscle through conditional deletion of the mitofusins Mfn1 and Mfn2, mitochondrial GTPases essential for fusion. Loss of the mitofusins causes severe mitochondrial dysfunction, compensatory mitochondrial proliferation, and muscle atrophy. Mutant mice have severe mtDNA depletion in muscle that precedes physiological abnormalities. Moreover, the mitochondrial genomes of the mutant muscle rapidly accumulate point mutations and deletions. In a related experiment, we find that disruption of mitochondrial fusion strongly increases mitochondrial dysfunction and lethality in a mouse model with high levels of mtDNA mutations. With its dual function in safeguarding mtDNA integrity and preserving mtDNA function in the face of mutations, mitochondrial fusion is likely to be a protective factor in human disorders associated with mtDNA mutations.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

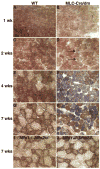
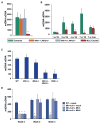
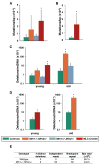
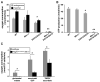
Comment in
-
Organelle dynamics: Fusing for stability.Nat Rev Mol Cell Biol. 2010 Jun;11(6):391. doi: 10.1038/nrm2910. Epub 2010 May 12. Nat Rev Mol Cell Biol. 2010. PMID: 20461095 No abstract available.
References
-
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007a;8:870–879. - PubMed
-
- Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503–536. - PubMed
-
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. - PubMed
-
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

