Antimicrobial peptides in toroidal and cylindrical pores
- PMID: 20403332
- PMCID: PMC2885466
- DOI: 10.1016/j.bbamem.2010.04.004
Antimicrobial peptides in toroidal and cylindrical pores
Abstract
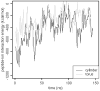
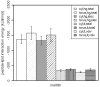
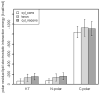
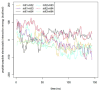
Antimicrobial peptides (AMPs) are small, usually cationic peptides, which permeabilize biological membranes. Their mechanism of action is still not well understood. Here we investigate the preference of alamethicin and melittin for pores of different shapes, using molecular dynamics (MD) simulations of the peptides in pre-formed toroidal and cylindrical pores. When an alamethicin hexamer is initially embedded in a cylindrical pore, at the end of the simulation the pore remains cylindrical or closes if glutamines in the N-termini are not located within the pore. On the other hand, when a melittin tetramer is embedded in toroidal pore or in a cylindrical pore, at the end of the simulation the pore is lined both with peptides and lipid headgroups, and, thus, can be classified as a toroidal pore. These observations agree with the prevailing views that alamethicin forms barrel-stave pores whereas melittin forms toroidal pores. Both alamethicin and melittin form amphiphilic helices in the presence of membranes, but their net charge differs; at pH approximately 7, the net charge of alamethicin is -1 whereas that of melittin is +5. This gives rise to stronger electrostatic interactions of melittin with membranes than those of alamethicin. The melittin tetramer interacts more strongly with lipids in the toroidal pore than in the cylindrical one, due to more favorable electrostatic interactions.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Charge distribution and imperfect amphipathicity affect pore formation by antimicrobial peptides.Biochim Biophys Acta. 2012 May;1818(5):1274-83. doi: 10.1016/j.bbamem.2012.01.016. Epub 2012 Jan 25. Biochim Biophys Acta. 2012. PMID: 22290189 Free PMC article.
-
Antimicrobial peptides bind more strongly to membrane pores.Biochim Biophys Acta. 2010 Aug;1798(8):1494-502. doi: 10.1016/j.bbamem.2010.02.023. Epub 2010 Feb 24. Biochim Biophys Acta. 2010. PMID: 20188066 Free PMC article.
-
Barrel-stave model or toroidal model? A case study on melittin pores.Biophys J. 2001 Sep;81(3):1475-85. doi: 10.1016/S0006-3495(01)75802-X. Biophys J. 2001. PMID: 11509361 Free PMC article.
-
Simulation studies of the interaction of antimicrobial peptides and lipid bilayers.Biochim Biophys Acta. 1999 Dec 15;1462(1-2):185-200. doi: 10.1016/s0005-2736(99)00206-0. Biochim Biophys Acta. 1999. PMID: 10590308 Review.
-
Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin.J Membr Biol. 1997 Apr 1;156(3):197-211. doi: 10.1007/s002329900201. J Membr Biol. 1997. PMID: 9096062 Review. No abstract available.
Cited by
-
Comparative functional properties of engineered cationic antimicrobial peptides consisting exclusively of tryptophan and either lysine or arginine.J Med Microbiol. 2016 Jun;65(6):554-565. doi: 10.1099/jmm.0.000258. Epub 2016 Apr 5. J Med Microbiol. 2016. PMID: 27046192 Free PMC article.
-
Inhibition of Tolaasin Cytotoxicity Causing Brown Blotch Disease in Cultivated Mushrooms Using Tolaasin Inhibitory Factors.Toxins (Basel). 2023 Jan 12;15(1):66. doi: 10.3390/toxins15010066. Toxins (Basel). 2023. PMID: 36668885 Free PMC article.
-
Interaction of Uperin Peptides with Model Membranes: Molecular Dynamics Study.Membranes (Basel). 2023 Mar 23;13(4):370. doi: 10.3390/membranes13040370. Membranes (Basel). 2023. PMID: 37103797 Free PMC article.
-
Intestinal biofilms: pathophysiological relevance, host defense, and therapeutic opportunities.Clin Microbiol Rev. 2024 Sep 12;37(3):e0013323. doi: 10.1128/cmr.00133-23. Epub 2024 Jul 12. Clin Microbiol Rev. 2024. PMID: 38995034 Free PMC article. Review.
-
Transmembrane Pore Structures of β-Hairpin Antimicrobial Peptides by All-Atom Simulations.J Phys Chem B. 2017 Oct 5;121(39):9126-9140. doi: 10.1021/acs.jpcb.7b06591. Epub 2017 Sep 21. J Phys Chem B. 2017. PMID: 28879767 Free PMC article.
References
-
- Hancock REW. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis. 2001;1:156–164. - PubMed
-
- Brown KL, Hancock REW. Cationic host defense (antimicrobial) peptides. Curr Opin Immunol. 2006;18:24–30. - PubMed
-
- Brogden K. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. - PubMed
-
- Bechinger B. Structure and function of membrane-lytic peptides. Crit Rev Plant Sci. 2004;23:271–292.
-
- Cole AM. Antimicrobial peptide microbicides targeting HIV. Protein Pept Lett. 2005;12:41–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

