Drosophila UNC-45 prevents heat-induced aggregation of skeletal muscle myosin and facilitates refolding of citrate synthase
- PMID: 20403336
- PMCID: PMC2888609
- DOI: 10.1016/j.bbrc.2010.04.090
Drosophila UNC-45 prevents heat-induced aggregation of skeletal muscle myosin and facilitates refolding of citrate synthase
Abstract
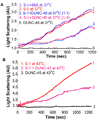
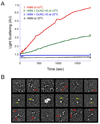
UNC-45 belongs to the UCS (UNC-45, CRO1, She4p) domain protein family, whose members interact with various classes of myosin. Here we provide structural and biochemical evidence that Escherichia coli-expressed Drosophila UNC-45 (DUNC-45) maintains the integrity of several substrates during heat-induced stress in vitro. DUNC-45 displays chaperone function in suppressing aggregation of the muscle myosin heavy meromyosin fragment, the myosin S-1 motor domain, alpha-lactalbumin and citrate synthase. Biochemical evidence is supported by electron microscopy, which reveals the first structural evidence that DUNC-45 prevents inter- or intra-molecular aggregates of skeletal muscle heavy meromyosin caused by elevated temperatures. We also demonstrate for the first time that UNC-45 is able to refold a denatured substrate, urea-unfolded citrate synthase. Overall, this in vitro study provides insight into the fate of muscle myosin under stress conditions and suggests that UNC-45 protects and maintains the contractile machinery during in vivo stress.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Drosophila UNC-45 accumulates in embryonic blastoderm and in muscles, and is essential for muscle myosin stability.J Cell Sci. 2011 Mar 1;124(Pt 5):699-705. doi: 10.1242/jcs.078964. Epub 2011 Feb 1. J Cell Sci. 2011. PMID: 21285246 Free PMC article.
-
The UNC-45 chaperone is critical for establishing myosin-based myofibrillar organization and cardiac contractility in the Drosophila heart model.PLoS One. 2011;6(7):e22579. doi: 10.1371/journal.pone.0022579. Epub 2011 Jul 25. PLoS One. 2011. PMID: 21799905 Free PMC article.
-
The UNC-45 myosin chaperone: from worms to flies to vertebrates.Int Rev Cell Mol Biol. 2014;313:103-44. doi: 10.1016/B978-0-12-800177-6.00004-9. Int Rev Cell Mol Biol. 2014. PMID: 25376491 Free PMC article. Review.
-
Tracking UNC-45 chaperone-myosin interaction with a titin mechanical reporter.Biophys J. 2012 May 2;102(9):2212-9. doi: 10.1016/j.bpj.2012.03.013. Biophys J. 2012. PMID: 22824286 Free PMC article.
-
The UCS family of myosin chaperones.J Cell Sci. 2002 Nov 1;115(Pt 21):3983-90. doi: 10.1242/jcs.00107. J Cell Sci. 2002. PMID: 12356904 Review.
Cited by
-
X-ray crystal structure of the UCS domain-containing UNC-45 myosin chaperone from Drosophila melanogaster.Structure. 2011 Mar 9;19(3):397-408. doi: 10.1016/j.str.2011.01.002. Structure. 2011. PMID: 21397190 Free PMC article.
-
Chromatin occupancy patterns of the ETS repressor Yan: a mechanism for buffering gene expression against noise?Fly (Austin). 2013 Apr-Jun;7(2):92-8. doi: 10.4161/fly.24162. Epub 2013 Apr 1. Fly (Austin). 2013. PMID: 23575308 Free PMC article.
-
Mutational Analysis of the Structure and Function of the Chaperoning Domain of UNC-45B.Biophys J. 2020 Aug 18;119(4):780-791. doi: 10.1016/j.bpj.2020.07.012. Epub 2020 Jul 22. Biophys J. 2020. PMID: 32755562 Free PMC article.
-
UFD-2 is an adaptor-assisted E3 ligase targeting unfolded proteins.Nat Commun. 2018 Feb 2;9(1):484. doi: 10.1038/s41467-018-02924-7. Nat Commun. 2018. PMID: 29396393 Free PMC article.
-
UNC-45/CRO1/She4p (UCS) protein forms elongated dimer and joins two myosin heads near their actin binding region.Proc Natl Acad Sci U S A. 2010 Dec 14;107(50):21382-7. doi: 10.1073/pnas.1013038107. Epub 2010 Nov 29. Proc Natl Acad Sci U S A. 2010. PMID: 21115842 Free PMC article.
References
-
- Barral JM, Hutagalung AH, Brinker A, Hartl FU, Epstein HF. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 2002;295:669–671. - PubMed
-
- Kim J, Lowe T, Hoppe T. Protein quality control gets muscle into shape. Trends Cell. Biol. 2008;18:264–272. - PubMed
-
- Wong KC, Naqvi NI, Iino Y, Yamamoto M, Balasubramanian MK. Fission yeast Rng3p: an UCS-domain protein that mediates myosin II assembly during cytokinesis. J. Cell Sci. 2000;113:2421–2432. - PubMed
-
- Epstein HF, Thomson JN. Temperature-sensitive mutation affecting myofilament assembly in Caenorhabditis elegans. Nature. 1974;250:579–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

