Drosophila UNC-45 prevents heat-induced aggregation of skeletal muscle myosin and facilitates refolding of citrate synthase
- PMID: 20403336
- PMCID: PMC2888609
- DOI: 10.1016/j.bbrc.2010.04.090
Drosophila UNC-45 prevents heat-induced aggregation of skeletal muscle myosin and facilitates refolding of citrate synthase
Abstract
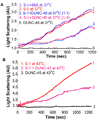
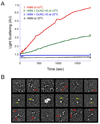
UNC-45 belongs to the UCS (UNC-45, CRO1, She4p) domain protein family, whose members interact with various classes of myosin. Here we provide structural and biochemical evidence that Escherichia coli-expressed Drosophila UNC-45 (DUNC-45) maintains the integrity of several substrates during heat-induced stress in vitro. DUNC-45 displays chaperone function in suppressing aggregation of the muscle myosin heavy meromyosin fragment, the myosin S-1 motor domain, alpha-lactalbumin and citrate synthase. Biochemical evidence is supported by electron microscopy, which reveals the first structural evidence that DUNC-45 prevents inter- or intra-molecular aggregates of skeletal muscle heavy meromyosin caused by elevated temperatures. We also demonstrate for the first time that UNC-45 is able to refold a denatured substrate, urea-unfolded citrate synthase. Overall, this in vitro study provides insight into the fate of muscle myosin under stress conditions and suggests that UNC-45 protects and maintains the contractile machinery during in vivo stress.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures

References
-
- Barral JM, Hutagalung AH, Brinker A, Hartl FU, Epstein HF. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 2002;295:669–671. - PubMed
-
- Kim J, Lowe T, Hoppe T. Protein quality control gets muscle into shape. Trends Cell. Biol. 2008;18:264–272. - PubMed
-
- Wong KC, Naqvi NI, Iino Y, Yamamoto M, Balasubramanian MK. Fission yeast Rng3p: an UCS-domain protein that mediates myosin II assembly during cytokinesis. J. Cell Sci. 2000;113:2421–2432. - PubMed
-
- Epstein HF, Thomson JN. Temperature-sensitive mutation affecting myofilament assembly in Caenorhabditis elegans. Nature. 1974;250:579–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

