Prolonged analgesic response of cornea to topical resiniferatoxin, a potent TRPV1 agonist
- PMID: 20403666
- PMCID: PMC2913152
- DOI: 10.1016/j.pain.2010.03.024
Prolonged analgesic response of cornea to topical resiniferatoxin, a potent TRPV1 agonist
Abstract
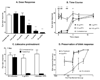
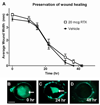
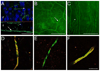
Analgesics currently available for the treatment of pain following ophthalmic surgery or injury are limited by transient effectiveness and undesirable or adverse side effects. The cornea is primarily innervated by small-diameter C-fiber sensory neurons expressing TRPV1 (transient receptor potential channel, subfamily V, member 1), a sodium/calcium cation channel expressed abundantly by nociceptive neurons and consequently a target for pain control. Resiniferatoxin (RTX), a potent TRPV1 agonist, produces transient analgesia when injected peripherally by inactivating TRPV1-expressing nerve terminals through excessive calcium influx. The aim of the present study was to evaluate topical RTX as a corneal analgesic. In rat cornea, a single application of RTX dose dependently eliminated or reduced the capsaicin eye wipe response for 3-5 days, with normal nociceptive responses returning by 5-7 days. RTX alone produced a brief but intense noxious response, similar to capsaicin, necessitating pretreatment of the cornea with a local anesthetic. Topical lidocaine, applied prior to RTX, blocks acute nociceptive responses to RTX without impairing the subsequent analgesic effect. Importantly, RTX analgesia (a) did not impair epithelial wound healing, (b) left the blink reflex intact and (c) occurred without detectable histological damage to the cornea. Immunohistochemistry showed that loss of CGRP immunoreactivity, a surrogate marker for TRPV1-expressing fibers, extended at least to the corneal-scleral boundary and displayed a progressive return, coincident with the return of capsaicin sensitivity. These data suggest that RTX may be a safe and effective treatment for post-operative or post-injury ophthalmic pain.
Conflict of interest statement
Conflict of Interest Statement. We have no financial or other conflicts of interest.
Figures

References
-
- Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, Olah Z, Mannes AJ. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103:1052–1059. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Caudle RM, Karai L, Mena N, Cooper BY, Mannes AJ, Perez FM, Iadarola MJ, Olah Z. Resiniferatoxin induced loss of plasma membrane in vanilloid receptor expressing cells. J. Neurotoxicology. 2003;24:895–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

