Non-classical actions of testosterone and spermatogenesis
- PMID: 20403869
- PMCID: PMC2871922
- DOI: 10.1098/rstb.2009.0258
Non-classical actions of testosterone and spermatogenesis
Abstract
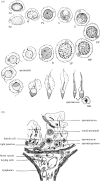
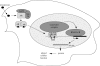
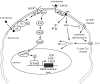
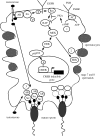
Testosterone is essential to maintain spermatogenesis and male fertility. In the absence of testosterone stimulation, spermatogenesis does not proceed beyond the meiosis stage. After withdrawal of testosterone, germ cells that have progressed beyond meiosis detach from supporting Sertoli cells and die, whereas mature sperm cannot be released from Sertoli cells resulting in infertility. The classical mechanism of testosterone action in which testosterone activates gene transcription by causing the androgen receptor to translocate to and bind specific DNA regulatory elements does not appear to fully explain testosterone regulation of spermatogenesis. This review discusses two non-classical testosterone signalling pathways in Sertoli cells and their potential effects on spermatogenesis. Specifically, testosterone-mediated activation of phospholipase C and calcium influx into Sertoli cells is described. Also, testosterone activation of Src, EGF receptor and ERK kinases as well as the activation of the CREB transcription factor and CREB-mediated transcription is reviewed. Regulation of germ cell adhesion to Sertoli cells and release of mature sperm from Sertoli cells by kinases regulated by the non-classical testosterone pathway is discussed. The evidence accumulated suggests that classical and non-classical testosterone signalling contribute to the maintenance of spermatogenesis and male fertility.
Figures
References
-
- Awoniyi C. A., Santulli R., Sprando R. L., Ewing L. L., Zirkin B. R.1989Restoration of advanced spermatogenic cells in the experimentally regressed rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology 124, 1217–1223 (doi:10.1210/endo-124-3-1217) - DOI - PubMed
-
- Beardsley A., O'Donnell L.2003Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol. Reprod. 68, 1299–1307 (doi:10.1095/biolreprod.102.009811) - DOI - PubMed
-
- Bremner W. J., Millar M. R., Sharpe R. M., Saunders P. T. K.1994Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology 135, 1227–1234 (doi:10.1210/en.135.3.1227) - DOI - PubMed
-
- Chang C., Chen Y. T., Yeh S. D., Xu Q., Wang R. S., Guillou F., Lardy H., Yeh S.2004Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl Acad. Sci. USA 101, 6876–6881 (doi:10.1073/pnas.0307306101) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

