Unitary inhibitory field potentials in the CA3 region of rat hippocampus
- PMID: 20403979
- PMCID: PMC2911213
- DOI: 10.1113/jphysiol.2009.185918
Unitary inhibitory field potentials in the CA3 region of rat hippocampus
Abstract
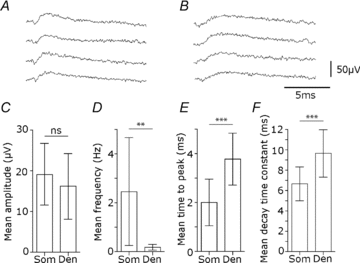
Glickfeld and colleagues (2009) suggested that single hippocampal interneurones generate field potentials at monosynaptic latencies. We pursued this observation in simultaneous intracellular and multiple extracellular records from the CA3 region of rat hippocampal slices. We confirmed that interneurones evoked field potentials at monosynaptic latencies. Pyramidal cells initiated disynaptic inhibitory field potentials, but did not initiate detectable monosynaptic excitatory fields. We confirmed that inhibitory fields were GABAergic in nature and showed they were suppressed at low external Cl(-), suggesting they originate at postsynaptic sites. Field potentials generated by a single interneuron were detected at multiple sites over distances of more than 800 mum along the stratum pyramidale of the CA3 region. We used arrays of extracellular electrodes to examine amplitude distributions of spontaneous inhibitory fields recorded at sites orthogonal to or along the CA3 stratum pyramidale. Cluster analysis of spatially distributed inhibitory field events let us separate events generated by interneurones terminating on distinct zones of somato-dendritic axis. Events generated at dendritic sites had similar amplitudes but occurred less frequently and had somewhat slower kinetics than perisomatic events generated near the stratum pyramidale. In records from multiple sites in the CA3 stratum pyramidale, we distinguished inhibitory fields that seemed to be initiated by interneurones with spatially distinct axonal arborisations.
Figures






Comment in
-
Currents in space: understanding inhibitory field potentials.J Physiol. 2010 Jun 15;588(Pt 12):2015-6. doi: 10.1113/jphysiol.2010.192443. J Physiol. 2010. PMID: 20551020 Free PMC article. No abstract available.
References
-
- Andersen P, Bliss TV, Skrede KK. Unit analysis of hippocampal population spikes. Exp Brain Res. 1971;13:208–221. - PubMed
-
- Brecht M, Schneider M, Sakmann B, Margrie TW. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature. 2004;427:704–710. - PubMed
-
- Biernacki C, Celeux G, Govaert G, Langrognet F. Model-based cluster and discriminant analysis with the MIXMOD software. Comput Stat Data Anal. 2006;51(2):587–600.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

