Rapid cytopathic effects of Clostridium perfringens beta-toxin on porcine endothelial cells
- PMID: 20404076
- PMCID: PMC2897397
- DOI: 10.1128/IAI.01284-09
Rapid cytopathic effects of Clostridium perfringens beta-toxin on porcine endothelial cells
Abstract
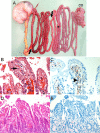
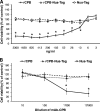
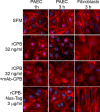
Clostridium perfringens type C isolates cause fatal, segmental necro-hemorrhagic enteritis in animals and humans. Typically, acute intestinal lesions result from extensive mucosal necrosis and hemorrhage in the proximal jejunum. These lesions are frequently accompanied by microvascular thrombosis in affected intestinal segments. In previous studies we demonstrated that there is endothelial localization of C. perfringens type C beta-toxin (CPB) in acute lesions of necrotizing enteritis. This led us to hypothesize that CPB contributes to vascular necrosis by directly damaging endothelial cells. By performing additional immunohistochemical studies using spontaneously diseased piglets, we confirmed that CPB binds to the endothelial lining of vessels showing early signs of thrombosis. To investigate whether CPB can disrupt the endothelium, we exposed primary porcine aortic endothelial cells to C. perfringens type C culture supernatants and recombinant CPB. Both treatments rapidly induced disruption of the actin cytoskeleton, cell border retraction, and cell shrinkage, leading to destruction of the endothelial monolayer in vitro. These effects were followed by cell death. Cytopathic and cytotoxic effects were inhibited by neutralization of CPB. Taken together, our results suggest that CPB-induced disruption of endothelial cells may contribute to the pathogenesis of C. perfringens type C enteritis.
Figures



References
-
- Albini, S., I. Brodard, A. Jaussi, N. Wollschlaeger, J. Frey, R. Miserez, and C. Abril. 2008. Real-time multiplex PCR assays for reliable detection of Clostridium perfringens toxin genes in animal isolates. Vet. Microbiol. 127:179-185. - PubMed
-
- Amimoto, K., T. Noro, E. Oishi, and M. Shimizu. 2007. A novel toxin homologous to large clostridial cytotoxins found in culture supernatant of Clostridium perfringens type C. Microbiology 153:1198-1206. - PubMed
-
- Bouis, D., G. A. Hospers, C. Meijer, G. Molema, and N. H. Mulder. 2001. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis 4:91-102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

