Dendritic cells inhibit the progression of Listeria monocytogenes intracellular infection by retaining bacteria in major histocompatibility complex class II-rich phagosomes and by limiting cytosolic growth
- PMID: 20404078
- PMCID: PMC2897391
- DOI: 10.1128/IAI.01027-09
Dendritic cells inhibit the progression of Listeria monocytogenes intracellular infection by retaining bacteria in major histocompatibility complex class II-rich phagosomes and by limiting cytosolic growth
Abstract
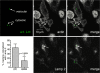
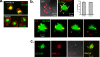
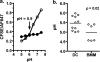
Dendritic cells (DC) provide a suboptimal niche for the growth of Listeria monocytogenes, a facultative intracellular bacterial pathogen of immunocompromised and pregnant hosts. This is due in part to a failure of large numbers of bacteria to escape to the cytosol, an essential step in the intracellular life cycle that is mediated by listeriolysin O (LLO). Here, we demonstrate that wild-type bacteria that failed to enter the cytosol of bone marrow-derived DC were retained in a LAMP2+ compartment. An isogenic L. monocytogenes strain that produces an LLO protein with reduced pore-forming activity had a severe escape and growth phenotype in DC. Few mutant bacteria entered the cytosol in the first 2 h and were instead found in LAMP2+, major histocompatibility complex class II+ (MHC-II+) H2-DM vesicles characteristic of MHC-II antigen loading compartments (MIIC). In contrast, the mutant had a minor phenotype in bone marrow-derived macrophages (BMM) despite the reduced LLO activity. In the first hour, DC phagosomes acidified to a pH that was, on average, half a point higher than that of BMM phagosomes. Unlike BMM, L. monocytogenes growth in DC was minimal after 5 h, and consequently, DC remained viable and matured late in infection. Taken together, the data are consistent with a model in which phagosomal maturation events associated with the acquisition of MHC-II molecules present a suboptimal environment for L. monocytogenes escape to the DC cytosol, possibly by limiting the activity of LLO. This, in combination with an undefined mechanism that controls bacterial growth late in infection, promotes DC survival during the critical maturation response.
Figures



Similar articles
-
Differential susceptibility of bone marrow-derived dendritic cells and macrophages to productive infection with Listeria monocytogenes.Cell Microbiol. 2007 Jun;9(6):1397-411. doi: 10.1111/j.1462-5822.2006.00880.x. Epub 2007 Jan 22. Cell Microbiol. 2007. PMID: 17250592
-
Antigen secreted from noncytosolic Listeria monocytogenes is processed by the classical MHC class I processing pathway.J Immunol. 1999 Jun 1;162(11):6341-50. J Immunol. 1999. PMID: 10352246
-
Cytolysin-dependent escape of the bacterium from the phagosome is required but not sufficient for induction of the Th1 immune response against Listeria monocytogenes infection: distinct role of Listeriolysin O determined by cytolysin gene replacement.Infect Immun. 2007 Aug;75(8):3791-801. doi: 10.1128/IAI.01779-06. Epub 2007 May 21. Infect Immun. 2007. PMID: 17517863 Free PMC article.
-
Why is Listeria monocytogenes such a potent inducer of CD8+ T-cells?Cell Microbiol. 2020 Apr;22(4):e13175. doi: 10.1111/cmi.13175. Cell Microbiol. 2020. PMID: 32185899 Free PMC article. Review.
-
Autophagy in immunity against intracellular bacteria.Curr Top Microbiol Immunol. 2009;335:189-215. doi: 10.1007/978-3-642-00302-8_9. Curr Top Microbiol Immunol. 2009. PMID: 19802566 Review.
Cited by
-
Production of IFN-γ by splenic dendritic cells during innate immune responses against Francisella tularensis LVS depends on MyD88, but not TLR2, TLR4, or TLR9.PLoS One. 2020 Aug 3;15(8):e0237034. doi: 10.1371/journal.pone.0237034. eCollection 2020. PLoS One. 2020. PMID: 32745117 Free PMC article.
-
Fascin confers resistance to Listeria infection in dendritic cells.J Immunol. 2013 Dec 15;191(12):6156-64. doi: 10.4049/jimmunol.1300498. Epub 2013 Nov 15. J Immunol. 2013. PMID: 24244012 Free PMC article.
-
The intact structural form of LLO in endosomes cannot protect against listeriosis.Int J Biochem Mol Biol. 2011;2(3):207-18. Epub 2011 Jul 15. Int J Biochem Mol Biol. 2011. PMID: 22003433 Free PMC article.
-
The influence of infectious factors on dendritic cell apoptosis.Arch Med Sci. 2015 Oct 12;11(5):1044-51. doi: 10.5114/aoms.2015.54860. Arch Med Sci. 2015. PMID: 26528349 Free PMC article.
-
Dendritic cells activate pyroptosis and effector-triggered apoptosis to restrict Legionella infection.mBio. 2025 Jul 9;16(7):e0125725. doi: 10.1128/mbio.01257-25. Epub 2025 Jun 18. mBio. 2025. PMID: 40530878 Free PMC article.
References
-
- Alberti-Segui, C., K. R. Goeden, and D. E. Higgins. 2007. Differential function of Listeria monocytogenes listeriolysin O and phospholipases C in vacuolar dissolution following cell-to-cell spread. Cell Microbiol. 9:179-195. - PubMed
-
- Aoshi, T., B. H. Zinselmeyer, V. Konjufca, J. N. Lynch, X. Zhang, Y. Koide, and M. J. Miller. 2008. Bacterial entry to the splenic white pulp initiates antigen presentation to CD8+ T cells. Immunity 29:476-486. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

