Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis
- PMID: 20404086
- PMCID: PMC2876685
- DOI: 10.1128/MCB.01428-09
Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis
Abstract
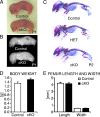
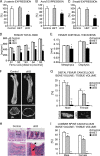
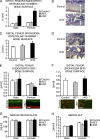
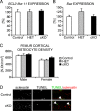
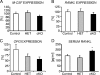
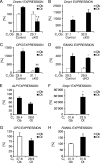
Beta-Catenin-dependent canonical Wnt signaling plays an important role in bone metabolism by controlling differentiation of bone-forming osteoblasts and bone-resorbing osteoclasts. To investigate its function in osteocytes, the cell type constituting the majority of bone cells, we generated osteocyte-specific beta-catenin-deficient mice (Ctnnb1(loxP/loxP); Dmp1-Cre). Homozygous mutants were born at normal Mendelian frequency with no obvious morphological abnormalities or detectable differences in size or body weight, but bone mass accrual was strongly impaired due to early-onset, progressive bone loss in the appendicular and axial skeleton with mild growth retardation and premature lethality. Cancellous bone mass was almost completely absent, and cortical bone thickness was dramatically reduced. The low-bone-mass phenotype was associated with increased osteoclast number and activity, whereas osteoblast function and osteocyte density were normal. Cortical bone Wnt/beta-catenin target gene expression was reduced, and of the known regulators of osteoclast differentiation, osteoprotegerin (OPG) expression was significantly downregulated in osteocyte bone fractions of mutant mice. Moreover, the OPG levels expressed by osteocytes were higher than or comparable to the levels expressed by osteoblasts during skeletal growth and at maturity, suggesting that the reduction in osteocytic OPG and the concomitant increase in osteocytic RANKL/OPG ratio contribute to the increased number of osteoclasts and resorption in osteocyte-specific beta-catenin mutants. Together, these results reveal a crucial novel function for osteocyte beta-catenin signaling in controlling bone homeostasis.
Figures

References
-
- Aguirre, J. I., L. I. Plotkin, S. A. Stewart, R. S. Weinstein, A. M. Parfitt, S. C. Manolagas, and T. Bellido. 2006. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 21:605-615. - PubMed
-
- Almeida, M., L. Han, M. Martin-Millan, C. A. O'Brien, and S. C. Manolagas. 2007. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J. Biol. Chem. 282:27298-27305. - PubMed
-
- Armstrong, V. J., M. Muzylak, A. Sunters, G. Zaman, L. K. Saxon, J. S. Price, and L. E. Lanyon. 2007. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J. Biol. Chem. 282:20715-20727. - PubMed
-
- Babij, P., W. Zhao, C. Small, Y. Kharode, P. J. Yaworsky, M. L. Bouxsein, P. S. Reddy, P. V. Bodine, J. A. Robinson, B. Bhat, J. Marzolf, R. A. Moran, and F. Bex. 2003. High bone mass in mice expressing a mutant LRP5 gene. J. Bone Miner. Res. 18:960-974. - PubMed
-
- Bennett, C. N., H. Ouyang, Y. L. Ma, Q. Zeng, I. Gerin, K. M. Sousa, T. F. Lane, V. Krishnan, K. D. Hankenson, and O. A. MacDougald. 2007. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J. Bone Miner. Res. 22:1924-1932. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
