TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo
- PMID: 20404094
- PMCID: PMC2876666
- DOI: 10.1128/MCB.00240-10
TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo
Abstract
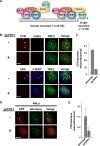
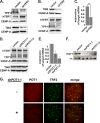
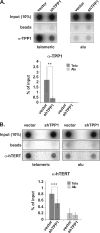
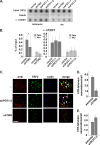
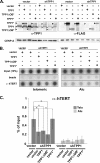
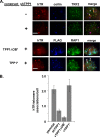
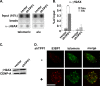
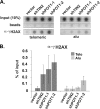
Recruitment to telomeres is a pivotal step in the function and regulation of human telomerase; however, the molecular basis for recruitment is not known. Here, we have directly investigated the process of telomerase recruitment via fluorescence in situ hybridization (FISH) and chromatin immunoprecipitation (ChIP). We find that depletion of two components of the shelterin complex that is found at telomeres--TPP1 and the protein that tethers TPP1 to the complex, TIN2--results in a loss of telomerase recruitment. On the other hand, we find that the majority of the observed telomerase association with telomeres does not require POT1, the shelterin protein that links TPP1 to the single-stranded region of the telomere. Deletion of the oligonucleotide/oligosaccharide binding fold (OB-fold) of TPP1 disrupts telomerase recruitment. In addition, while loss of TPP1 results in the appearance of DNA damage factors at telomeres, the DNA damage response per se does not account for the telomerase recruitment defect observed in the absence of TPP1. Our findings indicate that TIN2-anchored TPP1 plays a major role in the recruitment of telomerase to telomeres in human cells and that recruitment does not depend on POT1 or interaction of the shelterin complex with the single-stranded region of the telomere.
Figures
References
-
- Armanios, M. Y., J. J. Chen, J. D. Cogan, J. K. Alder, R. G. Ingersoll, C. Markin, W. E. Lawson, M. Xie, I. Vulto, J. A. Phillips III, P. M. Lansdorp, C. W. Greider, and J. E. Loyd. 2007. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 356:1317-1326. - PubMed
-
- Azzalin, C. M., and J. Lingner. 2006. The human RNA surveillance factor UPF1 is required for S phase progression and genome stability. Curr. Biol. 16:433-439. - PubMed
-
- Azzalin, C. M., P. Reichenbach, L. Khoriauli, E. Giulotto, and J. Lingner. 2007. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318:798-801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
