PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy
- PMID: 20404107
- PMCID: PMC2856912
- DOI: 10.1083/jcb.200910140
PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy
Abstract
Parkinson's disease (PD) is a prevalent neurodegenerative disorder. Recent identification of genes linked to familial forms of PD such as Parkin and PINK1 (PTEN-induced putative kinase 1) has revealed that ubiquitylation and mitochondrial integrity are key factors in disease pathogenesis. However, the exact mechanism underlying the functional interplay between Parkin-catalyzed ubiquitylation and PINK1-regulated mitochondrial quality control remains an enigma. In this study, we show that PINK1 is rapidly and constitutively degraded under steady-state conditions in a mitochondrial membrane potential-dependent manner and that a loss in mitochondrial membrane potential stabilizes PINK1 mitochondrial accumulation. Furthermore, PINK1 recruits Parkin from the cytoplasm to mitochondria with low membrane potential to initiate the autophagic degradation of damaged mitochondria. Interestingly, the ubiquitin ligase activity of Parkin is repressed in the cytoplasm under steady-state conditions; however, PINK1-dependent mitochondrial localization liberates the latent enzymatic activity of Parkin. Some pathogenic mutations of PINK1 and Parkin interfere with the aforementioned events, suggesting an etiological importance. These results provide crucial insight into the pathogenic mechanisms of PD.
Figures
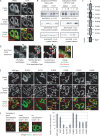
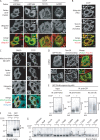
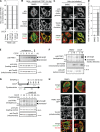
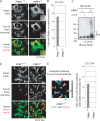
References
-
- Beilina A., Van Der Brug M., Ahmad R., Kesavapany S., Miller D.W., Petsko G.A., Cookson M.R. 2005. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc. Natl. Acad. Sci. USA. 102:5703–5708 10.1073/pnas.0500617102 - DOI - PMC - PubMed
-
- Exner N., Treske B., Paquet D., Holmström K., Schiesling C., Gispert S., Carballo-Carbajal I., Berg D., Hoepken H.H., Gasser T., et al. 2007. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J. Neurosci. 27:12413–12418 10.1523/JNEUROSCI.0719-07.2007 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

