Rho1 regulates apoptosis via activation of the JNK signaling pathway at the plasma membrane
- PMID: 20404112
- PMCID: PMC2856900
- DOI: 10.1083/jcb.200912010
Rho1 regulates apoptosis via activation of the JNK signaling pathway at the plasma membrane
Abstract
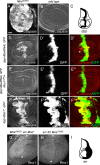
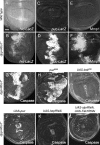
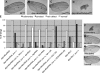
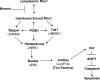
Precisely controlled growth and morphogenesis of developing epithelial tissues require coordination of multiple factors, including proliferation, adhesion, cell shape, and apoptosis. RhoA, a small GTPase, is known to control epithelial morphogenesis and integrity through its ability to regulate the cytoskeleton. In this study, we examine a less well-characterized RhoA function in cell survival. We demonstrate that the Drosophila melanogaster RhoA, Rho1, promotes apoptosis independently of Rho kinase through its effects on c-Jun NH(2)-terminal kinase (JNK) signaling. In addition, Rho1 forms a complex with Slipper (Slpr), an upstream activator of the JNK pathway. Loss of Moesin (Moe), an upstream regulator of Rho1 activity, results in increased levels of Rho1 at the plasma membrane and cortical accumulation of Slpr. Together, these results suggest that Rho1 functions at the cell cortex to regulate JNK activity and implicate Rho1 and Moe in epithelial cell survival.
Figures



Similar articles
-
The Cdc42/Par6/aPKC polarity complex regulates apoptosis-induced compensatory proliferation in epithelia.Curr Biol. 2010 Apr 27;20(8):677-86. doi: 10.1016/j.cub.2010.03.025. Epub 2010 Apr 8. Curr Biol. 2010. PMID: 20381350 Free PMC article.
-
Drosophila actin-Capping Protein limits JNK activation by the Src proto-oncogene.Oncogene. 2014 Apr 17;33(16):2027-39. doi: 10.1038/onc.2013.155. Epub 2013 May 6. Oncogene. 2014. PMID: 23644660
-
Impaired Hippo signaling promotes Rho1-JNK-dependent growth.Proc Natl Acad Sci U S A. 2015 Jan 27;112(4):1065-70. doi: 10.1073/pnas.1415020112. Epub 2015 Jan 12. Proc Natl Acad Sci U S A. 2015. PMID: 25583514 Free PMC article.
-
Pro-apoptotic and pro-proliferation functions of the JNK pathway of Drosophila: roles in cell competition, tumorigenesis and regeneration.Open Biol. 2019 Mar 29;9(3):180256. doi: 10.1098/rsob.180256. Open Biol. 2019. PMID: 30836847 Free PMC article. Review.
-
Regulation of Drosophila lifespan by JNK signaling.Exp Gerontol. 2011 May;46(5):349-54. doi: 10.1016/j.exger.2010.11.003. Epub 2010 Nov 25. Exp Gerontol. 2011. PMID: 21111799 Free PMC article. Review.
Cited by
-
The transmembrane protein Crumbs displays complex dynamics during follicular morphogenesis and is regulated competitively by Moesin and aPKC.Development. 2015 May 15;142(10):1869-78. doi: 10.1242/dev.115329. Epub 2015 Apr 29. Development. 2015. PMID: 25926360 Free PMC article.
-
Drosophila heat shock response requires the JNK pathway and phosphorylation of mixed lineage kinase at a conserved serine-proline motif.PLoS One. 2012;7(7):e42369. doi: 10.1371/journal.pone.0042369. Epub 2012 Jul 27. PLoS One. 2012. PMID: 22848763 Free PMC article.
-
The Hh pathway promotes cell apoptosis through Ci-Rdx-Diap1 axis.Cell Death Discov. 2021 Sep 24;7(1):263. doi: 10.1038/s41420-021-00653-3. Cell Death Discov. 2021. PMID: 34561426 Free PMC article.
-
STRIPAK complexes: structure, biological function, and involvement in human diseases.Int J Biochem Cell Biol. 2014 Feb;47:118-48. doi: 10.1016/j.biocel.2013.11.021. Epub 2013 Dec 11. Int J Biochem Cell Biol. 2014. PMID: 24333164 Free PMC article. Review.
-
The expression of moesin in astrocytoma: correlation with pathologic grade and poor clinical outcome.Med Oncol. 2013 Mar;30(1):372. doi: 10.1007/s12032-012-0372-z. Epub 2013 Jan 13. Med Oncol. 2013. PMID: 23315217
References
-
- Afshar K., Stuart B., Wasserman S.A. 2000. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development. 127:1887–1897 - PubMed
-
- Agnès F., Suzanne M., Noselli S. 1999. The Drosophila JNK pathway controls the morphogenesis of imaginal discs during metamorphosis. Development. 126:5453–5462 - PubMed
-
- Bloor J.W., Kiehart D.P. 2002. Drosophila RhoA regulates the cytoskeleton and cell-cell adhesion in the developing epidermis. Development. 129:3173–3183 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

