Convergence of Notch and beta-catenin signaling induces arterial fate in vascular progenitors
- PMID: 20404113
- PMCID: PMC2856895
- DOI: 10.1083/jcb.200904114
Convergence of Notch and beta-catenin signaling induces arterial fate in vascular progenitors
Erratum in
- J Cell Biol. 2013 Jul 8;202(1):179
Abstract
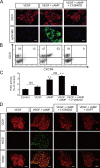
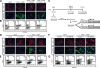
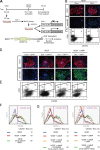
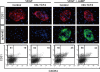
Molecular mechanisms controlling arterial-venous specification have not been fully elucidated. Previously, we established an embryonic stem cell differentiation system and demonstrated that activation of cAMP signaling together with VEGF induces arterial endothelial cells (ECs) from Flk1(+) vascular progenitor cells. Here, we show novel arterial specification machinery regulated by Notch and beta-catenin signaling. Notch and GSK3beta-mediated beta-catenin signaling were activated downstream of cAMP through phosphatidylinositol-3 kinase. Forced activation of Notch and beta-catenin with VEGF completely reconstituted cAMP-elicited arterial EC induction, and synergistically enhanced target gene promoter activity in vitro and arterial gene expression during in vivo angiogenesis. A protein complex with RBP-J, the intracellular domain of Notch, and beta-catenin was formed on RBP-J binding sites of arterial genes in arterial, but not venous ECs. This molecular machinery for arterial specification leads to an integrated and more comprehensive understanding of vascular signaling.
Figures



Similar articles
-
Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors.Arterioscler Thromb Vasc Biol. 2006 Sep;26(9):1977-84. doi: 10.1161/01.ATV.0000234978.10658.41. Epub 2006 Jun 29. Arterioscler Thromb Vasc Biol. 2006. PMID: 16809546
-
Roles of cyclic adenosine monophosphate signaling in endothelial cell differentiation and arterial-venous specification during vascular development.Circ J. 2011;75(2):253-60. doi: 10.1253/circj.cj-10-0915. Epub 2010 Dec 20. Circ J. 2011. PMID: 21178292 Review.
-
Angiopoietin-1/Tie2 signal augments basal Notch signal controlling vascular quiescence by inducing delta-like 4 expression through AKT-mediated activation of beta-catenin.J Biol Chem. 2011 Mar 11;286(10):8055-8066. doi: 10.1074/jbc.M110.192641. Epub 2011 Jan 6. J Biol Chem. 2011. PMID: 21212269 Free PMC article.
-
Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells.Mol Cell Biol. 2008 Dec;28(24):7427-41. doi: 10.1128/MCB.01962-07. Epub 2008 Oct 13. Mol Cell Biol. 2008. PMID: 18852283 Free PMC article.
-
Specification of arterial, venous, and lymphatic endothelial cells during embryonic development.Histol Histopathol. 2010 May;25(5):637-46. doi: 10.14670/HH-25.637. Histol Histopathol. 2010. PMID: 20238301 Free PMC article. Review.
Cited by
-
Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells.Stem Cells. 2013 Jan;31(1):92-103. doi: 10.1002/stem.1267. Stem Cells. 2013. PMID: 23079999 Free PMC article.
-
Stress-Induced Premature Senescence of Endothelial and Endothelial Progenitor Cells.Adv Pharmacol. 2016;77:281-306. doi: 10.1016/bs.apha.2016.04.007. Epub 2016 Jun 6. Adv Pharmacol. 2016. PMID: 27451101 Free PMC article. Review.
-
Human trabecular meshwork cells exhibit several characteristics of, but are distinct from, adipose-derived mesenchymal stem cells.J Ocul Pharmacol Ther. 2014 Mar-Apr;30(2-3):254-66. doi: 10.1089/jop.2013.0175. Epub 2014 Jan 23. J Ocul Pharmacol Ther. 2014. PMID: 24456002 Free PMC article.
-
Neuropilin-1 Mediated Arterial Differentiation of Murine Pluripotent Stem Cells.Stem Cells Dev. 2018 Apr 1;27(7):441-455. doi: 10.1089/scd.2017.0240. Epub 2018 Mar 13. Stem Cells Dev. 2018. PMID: 29415620 Free PMC article.
-
A Snail1/Notch1 signalling axis controls embryonic vascular development.Nat Commun. 2014 Jun 4;5:3998. doi: 10.1038/ncomms4998. Nat Commun. 2014. PMID: 24894949 Free PMC article.
References
-
- Adams R.H., Wilkinson G.A., Weiss C., Diella F., Gale N.W., Deutsch U., Risau W., Klein R. 1999. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 13:295–306 10.1101/gad.13.3.295 - DOI - PMC - PubMed
-
- Cattelino A., Liebner S., Gallini R., Zanetti A., Balconi G., Corsi A., Bianco P., Wolburg H., Moore R., Oreda B., et al. 2003. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J. Cell Biol. 162:1111–1122 10.1083/jcb.200212157 - DOI - PMC - PubMed
-
- Christensen S., Kodoyianni V., Bosenberg M., Friedman L., Kimble J. 1996. lag-1, a gene required for lin-12 and glp-1 signaling in Caenorhabditis elegans, is homologous to human CBF1 and Drosophila Su(H). Development. 122:1373–1383 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

