Nitazoxanide inhibits biofilm formation by Staphylococcus epidermidis by blocking accumulation on surfaces
- PMID: 20404119
- PMCID: PMC2897279
- DOI: 10.1128/AAC.00901-09
Nitazoxanide inhibits biofilm formation by Staphylococcus epidermidis by blocking accumulation on surfaces
Abstract
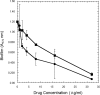
Coagulase-negative species of Staphylococcus are often associated with opportunistic hospital-acquired infections that arise from the colonization of indwelling catheters. Here we show that the antiparasitic drug nitazoxanide (NTZ) and its active metabolite, tizoxanide (TIZ), are inhibitory to the growth of Staphylococcus epidermidis and other staphylococci, including methicillin-resistant Staphylococcus aureus strains, under aerobic and microaerobic conditions (MICs, 8 to 16 microg/ml). At sub-MIC levels, NTZ and TIZ also inhibited biofilm production under static conditions by strains of S. epidermidis and Staphylococcus haemolyticus with a 50% inhibitory concentration of approximately 2.5 microg/ml (8 microM). The 5-nitro group was required for biological activity, and a hydrophilic derivative of NTZ (AMIX) also inhibited biofilm formation. NTZ did not disperse the existing biofilm but did block further accumulation. Sub-MICs of NTZ had no effect on primary attachment to surfaces at either 4 or 37 degrees C. The inhibitory action of NTZ and TIZ, but not vancomycin, on biofilm production could be reversed by the addition of zinc salts (2.5 to 40 microM) but not other metals, suggesting that NTZ might target the zinc-dependent accumulation-associated protein (Aap) that mediates accumulation on surfaces. However, neither NTZ nor TIZ formed chelation complexes with zinc salts, based on spectrophotometric and nuclear magnetic resonance analyses, and addition of excess zinc to NTZ-grown bacteria (apo-Aap) did not restore the accumulation phenotype. Our studies suggest that sub-MIC levels of NTZ may affect the assembly or function of cell structures associated with the biofilm phenotype.
Figures

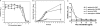


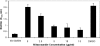
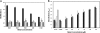
Similar articles
-
Nitazoxanide, a nitrothiazolide antiparasitic drug, is an anti-Helicobacter pylori agent with anti-vacuolating toxin activity.Chemotherapy. 1999 Jul-Aug;45(4):303-12. doi: 10.1159/000007200. Chemotherapy. 1999. PMID: 10394014
-
Antibacterial and anti-biofilm activities of thiazolidione derivatives against clinical staphylococcus strains.Emerg Microbes Infect. 2015 Jan;4(1):e1. doi: 10.1038/emi.2015.1. Epub 2015 Jan 7. Emerg Microbes Infect. 2015. PMID: 26038759 Free PMC article.
-
Comparison of linezolid and vancomycin lock solutions with and without heparin against biofilm-producing bacteria.Am J Health Syst Pharm. 2017 May 1;74(9):e193-e201. doi: 10.2146/ajhp150804. Am J Health Syst Pharm. 2017. PMID: 28438824
-
In vitro activities of telavancin and vancomycin against biofilm-producing Staphylococcus aureus, S. epidermidis, and Enterococcus faecalis strains.Antimicrob Agents Chemother. 2009 Jul;53(7):3166-9. doi: 10.1128/AAC.01642-08. Epub 2009 May 18. Antimicrob Agents Chemother. 2009. PMID: 19451302 Free PMC article.
-
Nitazoxanide inhibits biofilm production and hemagglutination by enteroaggregative Escherichia coli strains by blocking assembly of AafA fimbriae.Antimicrob Agents Chemother. 2010 Apr;54(4):1526-33. doi: 10.1128/AAC.01279-09. Epub 2010 Jan 19. Antimicrob Agents Chemother. 2010. PMID: 20086145 Free PMC article.
Cited by
-
Efficacy of antiamebic drugs in a mouse model.Am J Trop Med Hyg. 2011 Apr;84(4):581-6. doi: 10.4269/ajtmh.2011.10-0580. Am J Trop Med Hyg. 2011. PMID: 21460014 Free PMC article.
-
Preclinical studies of amixicile, a systemic therapeutic developed for treatment of Clostridium difficile infections that also shows efficacy against Helicobacter pylori.Antimicrob Agents Chemother. 2014 Aug;58(8):4703-12. doi: 10.1128/AAC.03112-14. Epub 2014 Jun 2. Antimicrob Agents Chemother. 2014. PMID: 24890599 Free PMC article.
-
Melittin Inhibition and Eradication Activity for Resistant Polymicrobial Biofilm Isolated from a Dairy Industry after Disinfection.Int J Microbiol. 2019 Jan 15;2019:4012394. doi: 10.1155/2019/4012394. eCollection 2019. Int J Microbiol. 2019. PMID: 30766602 Free PMC article.
-
A review on possible mechanistic insights of Nitazoxanide for repurposing in COVID-19.Eur J Pharmacol. 2021 Jan 15;891:173748. doi: 10.1016/j.ejphar.2020.173748. Epub 2020 Nov 20. Eur J Pharmacol. 2021. PMID: 33227285 Free PMC article. Review.
-
Rsp inhibits attachment and biofilm formation by repressing fnbA in Staphylococcus aureus MW2.J Bacteriol. 2011 Oct;193(19):5231-41. doi: 10.1128/JB.05454-11. Epub 2011 Jul 29. J Bacteriol. 2011. PMID: 21804010 Free PMC article.
References
-
- Cerca, N., S. Martins, S. Sillankorva, K. K. Jefferson, G. B. Pier, R. Oliverira, and J. Azeredo. 2005. Effects of growth in the presence of subinhibitory concentrations of dicloxacillin on Staphylococcus epidermidis and Staphylococcus haemolyticus biofilms. Appl. Environ. Microbiol. 71:8677-8682. - PMC - PubMed
-
- Corrigan, R. M., D. Rigby, P. Handley, and T. J. Foster. 2007. The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology 153:2435-2446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

