Effects of ravuconazole treatment on parasite load and immune response in dogs experimentally infected with Trypanosoma cruzi
- PMID: 20404124
- PMCID: PMC2897273
- DOI: 10.1128/AAC.01742-09
Effects of ravuconazole treatment on parasite load and immune response in dogs experimentally infected with Trypanosoma cruzi
Abstract
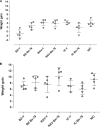
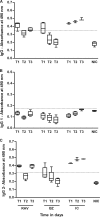
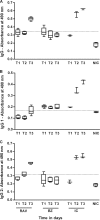
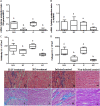
In this work, we investigated the in vivo activity of ravuconazole against the Y and Berenice-78 Trypanosoma cruzi strains using acutely infected dogs as hosts. Ravuconazole was well tolerated, as no significant side effects were observed during the treatment using 6.0 mg/kg twice a day (12 mg/kg/day) for up to 90 days. In all treated animals, parasitemia was permanently suppressed by the first day of treatment, independently of the parasite strain. Cultures of blood obtained posttreatment were negative for 90% of the animals, confirming that the drug induced a marked reduction in the parasite load. The results of PCR tests for T. cruzi in blood performed 1 month posttreatment were consistently negative for three of five and two of five animals infected with the Y and Berenice-78 strains, respectively. All ravuconazole-treated dogs consistently had negative serological test results during and until 30 days after treatment, regardless of the therapeutic scheme used. However, after the end of treatment, an increase in specific antibody levels was observed in all treated animals, although the antibody levels were always significantly lower than those of the nontreated control dogs. Despite being unable to induce a parasitological cure, ravuconazole treatment led to significant reductions in the levels of gamma interferon expression and lesions in cardiac tissues in animals infected with the Y strain, while the level of interleukin-10 mRNA expression increased. We conclude that ravuconazole has potent suppressive but not curative activity in the canine model of acute Chagas' disease, probably due to its unfavorable pharmacokinetic properties (half-life, 8.8 h). The longer half-life of ravuconazole in humans (4 to 8 days) makes it a promising drug for assessment for use as chemotherapy in human Chagas' disease.
Figures
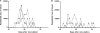

References
-
- Braga, M. S., L. Lauria-Pires, E. R. Argañaraz, R. J. Nascimento, and A. R. L. Teixeira. 2000. Persistent infections in chronic Chagas' disease patients treated with anti-Trypanosoma cruzi nitroderivatives. Rev. Inst. Med. Trop. Sao Paulo 42:157-161. - PubMed
-
- Britto, C., M. A. Cardoso, P. Wincker, and C. M. Morel. 1993. A simple protocol for the physical cleavage of Trypanosoma cruzi kinetoplast DNA present in blood samples and its use in polymerase chain reaction (PCR)-based diagnosis of chronic Chagas disease. Mem. Inst. Oswaldo Cruz 88:171-172. - PubMed
-
- Caldas, I. S., A. Talvani, S. Caldas, C. M. Carneiro, M. Lana, P. M. Guedes, and M. T. Bahia. 2008. Benznidazole therapy during acute phase of Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitol. Res. 103:413-421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

