Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment
- PMID: 20404138
- PMCID: PMC2889597
- DOI: 10.1073/pnas.0911378107
Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment
Abstract
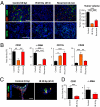
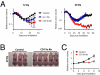
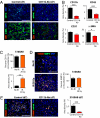
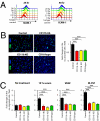
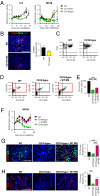
Despite recent advances in radiotherapy, loco-regional failures are still the leading cause of death in many cancer patients. We have previously reported that bone marrow-derived CD11b(+) myeloid cells are recruited to tumors grown in irradiated tissues, thereby restoring the vasculature and tumor growth. In this study, we examined whether neutralizing CD11b monoclonal antibodies could inhibit the recruitment of myeloid cells into irradiated tumors and inhibit their regrowth. We observed a significant enhancement of antitumor response to radiation in squamous cell carcinoma xenografts in mice when CD11b antibodies are administered systemically. Histological examination of tumors revealed that CD11b antibodies reduced infiltration of myeloid cells expressing S100A8 and matrix metalloproteinase-9. CD11b antibodies further inhibited bone marrow-derived cell adhesion and transmigration to C166 endothelial cell monolayers and chemotactic stimuli, respectively, to levels comparable to those from CD11b knockout or CD18 hypomorphic mice. Given the clinical availability of humanized CD18 antibodies, we tested two murine tumor models in CD18 hypomorphic or CD11b knockout mice and found that tumors were more sensitive to irradiation when grown in CD18 hypomorphic mice but not in CD11b knockout mice. When CD18 hypomorphism was partially rescued by reconstitution with the wild-type bone marrow, the resistance of the tumors to irradiation was restored. Our study thus supports the rationale of using clinically available Mac-1 (CD11b/CD18) antibodies as an adjuvant therapy to radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Verellen D, et al. Innovations in image-guided radiotherapy. Nat Rev Cancer. 2007;7:949–960. - PubMed
-
- Cummings B, et al. Five year results of a randomized trial comparing hyperfractionated to conventional radiotherapy over four weeks in locally advanced head and neck cancer. Radiother Oncol. 2007;85:7–16. - PubMed
-
- Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–158. - PubMed
-
- Shojaei F, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

