Esophageal cancer-related gene 4 is a secreted inducer of cell senescence expressed by aged CNS precursor cells
- PMID: 20404145
- PMCID: PMC2889548
- DOI: 10.1073/pnas.0911446107
Esophageal cancer-related gene 4 is a secreted inducer of cell senescence expressed by aged CNS precursor cells
Abstract
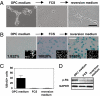
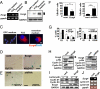
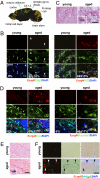
Mammalian aging is thought to be partially caused by the diminished capacity of stem/precursor cells to undergo self-renewing divisions. Although many cell-cycle regulators are involved in this process, it is unknown to what extent cell senescence, first identified as irreversible growth arrest in vitro, contributes to the aging process. Here, using a serum-induced mouse oligodendrocyte precursor cell (mOPC) senescence model, we identified esophageal cancer-related gene 4 (Ecrg4) as a senescence inducer with implications for the senescence-like state of postmitotic cells in the aging brain. Although mOPCs could proliferate indefinitely when cultured using the appropriate medium (OPC medium), they became senescent in the presence of serum and maintained their senescent phenotype even when the serum was subsequently replaced by OPC medium. We show that Ecrg4 was up-regulated in the senescent OPCs, its overexpression in OPCs induced senescence by accelerating the proteasome-dependent degradation of cyclins D1 and D3, and that its knockdown by a specific short hairpin RNA prevented these phenotypes. We also show that senescent OPCs secreted Ecrg4 and that recombinant Ecrg4 induced OPC senescence in culture. Moreover, increased Ecrg4 expression was observed in the OPCs and neural precursor cells in the aged mouse brain; this was accompanied by the expression of senescence-associated beta-galactosidase activity, indicating the cells' entrance into senescence. These results suggest that Ecrg4 is a factor linking neural-cell senescence and aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

