Natural immunity to pluripotency antigen OCT4 in humans
- PMID: 20404147
- PMCID: PMC2889362
- DOI: 10.1073/pnas.0915086107
Natural immunity to pluripotency antigen OCT4 in humans
Abstract
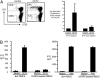
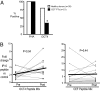
OCT4 is a transcription factor critical for the pluripotency of human embryonal stem (ES) and induced pluipotency stem (IPS) cells. OCT4 is commonly expressed in germ-cell tumors as well as putative cancer stem cells in several tumors, and is a key determinant of oncogenic fate in germ-cell tumors. The capacity of the human immune system to recognize this critical stem-cell gene is not known, but has implications for preventing tumors with ES/IPS-based therapies and targeting stem-cell pathways in cancer. Here we show that OCT4-specific T cells can be readily detected in freshly isolated T cells from most (>80%) healthy donors. The reactivity to OCT4-derived peptides resides primarily in the CD45RO(+) memory T-cell compartment and consists predominantly of CD4(+) T cells. T cells reactive against OCT4-derived peptides can be readily expanded in culture using peptide-loaded dendritic cells. In contrast to healthy donors, immunity to OCT4 was detected in only 35% of patients with newly diagnosed germ-cell tumors. However, chemotherapy of germ-cell tumors led to the induction of anti-OCT4 immunity in vivo in patients lacking such responses at baseline. These data demonstrate the surprising lack of immune tolerance to this critical pluripotency antigen in humans. Harnessing natural immunity to this antigen may allow immune-based targeting of pluripotency-related pathways for prevention of cancers, including those in the setting of ES/IPS-based therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
OCT4: biological functions and clinical applications as a marker of germ cell neoplasia.J Pathol. 2007 Jan;211(1):1-9. doi: 10.1002/path.2105. J Pathol. 2007. PMID: 17117392 Review.
-
OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma.Am J Surg Pathol. 2004 Jul;28(7):935-40. doi: 10.1097/00000478-200407000-00014. Am J Surg Pathol. 2004. PMID: 15223965
-
Spontaneous and therapy-induced immunity to pluripotency genes in humans: clinical implications, opportunities and challenges.Cancer Immunol Immunother. 2011 Mar;60(3):413-8. doi: 10.1007/s00262-010-0944-8. Epub 2010 Nov 23. Cancer Immunol Immunother. 2011. PMID: 21104412 Free PMC article. Review.
-
Oct4-induced pluripotency in adult neural stem cells.Cell. 2009 Feb 6;136(3):411-9. doi: 10.1016/j.cell.2009.01.023. Cell. 2009. PMID: 19203577
-
Rapid and efficient reprogramming of human amnion-derived cells into pluripotency by three factors OCT4/SOX2/NANOG.Differentiation. 2010 Sep-Oct;80(2-3):123-9. doi: 10.1016/j.diff.2010.03.002. Epub 2010 May 26. Differentiation. 2010. PMID: 20510497
Cited by
-
Human iPSC-Derived Renal Cells Change Their Immunogenic Properties during Maturation: Implications for Regenerative Therapies.Cells. 2022 Apr 13;11(8):1328. doi: 10.3390/cells11081328. Cells. 2022. PMID: 35456007 Free PMC article.
-
MHC-matched induced pluripotent stem cells can attenuate cellular and humoral immune responses but are still susceptible to innate immunity in pigs.PLoS One. 2014 Jun 13;9(6):e98319. doi: 10.1371/journal.pone.0098319. eCollection 2014. PLoS One. 2014. PMID: 24927426 Free PMC article.
-
Stem Cell Therapies for Treatment of Liver Disease.Biomedicines. 2016 Jan 6;4(1):2. doi: 10.3390/biomedicines4010002. Biomedicines. 2016. PMID: 28536370 Free PMC article. Review.
-
Immunogenicity of Tumor Initiating Stem Cells: Potential Applications in Novel Anticancer Therapy.Front Oncol. 2019 Apr 25;9:315. doi: 10.3389/fonc.2019.00315. eCollection 2019. Front Oncol. 2019. PMID: 31106150 Free PMC article. Review.
-
Strategies for Cancer Immunotherapy Using Induced Pluripotency Stem Cells-Based Vaccines.Cancers (Basel). 2020 Nov 30;12(12):3581. doi: 10.3390/cancers12123581. Cancers (Basel). 2020. PMID: 33266109 Free PMC article. Review.
References
-
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Yamanaka S. A fresh look at IPS cells. Cell. 2009;137:13–17. - PubMed
-
- Wang Y, Armstrong SA. Cancer: Inappropriate expression of stem cell programs? Cell Stem Cell. 2008;2:297–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

